-
PDF
- Split View
-
Views
-
Cite
Cite
Patrizia Trifilò, Piera M. Barbera, Fabio Raimondo, Andrea Nardini, Maria A. Lo Gullo, Coping with drought-induced xylem cavitation: coordination of embolism repair and ionic effects in three Mediterranean evergreens, Tree Physiology, Volume 34, Issue 2, February 2014, Pages 109–122, https://doi.org/10.1093/treephys/tpt119
Close - Share Icon Share
Abstract
Embolism repair and ionic effects on xylem hydraulic conductance have been documented in different tree species. However, the diurnal and seasonal patterns of both phenomena and their actual role in plants' responses to drought-induced xylem cavitation have not been thoroughly investigated. This study provides experimental evidence of the ability of three Mediterranean species to maintain hydraulic function under drought stress by coordinating the refilling of xylem conduits and ion-mediated enhancement of stem hydraulic conductance (Kstem). Vessel grouping indices and starch content in vessel-associated parenchyma cells were quantified to verify eventual correlations with ionic effects and refilling, respectively. Experiments were performed on stems of Ceratonia siliqua L., Olea europaea L. and Laurus nobilis L. Seasonal, ion-mediated changes in Kstem (ΔKstem) and diurnal and/or seasonal embolism repair were recorded for all three species, although with different temporal patterns. Field measurements of leaf specific stem hydraulic conductivity showed that it remained quite constant during the year, despite changes in the levels of embolism. Starch content in vessel-associated parenchyma cells changed on diurnal and seasonal scales in L. nobilis and O. europaea but not in C. siliqua. Values of ΔKstem were significantly correlated with vessel multiple fraction values (the ratio of grouped vessels to total number of vessels). Our data suggest that the regulation of xylem water transport in Mediterranean plants relies on a close integration between xylem refilling and ionic effects. These functional traits apparently play important roles in plants' responses to drought-induced xylem cavitation.
Introduction
Long-distance water transport in vascular plants occurs through a highly efficient conduit network, the xylem. According to the cohesion–tension theory, water is transported in the xylem under tension, i.e., in a metastable state (Tyree and Zimmermann 2002). Under such conditions, water is prone to cavitation, resulting in xylem embolism (Nardini et al. 2011b). Xylem embolism is a relatively common consequence of drought and freezing stress, and it can be detected even in well-watered plants (Choat et al. 2012). When embolism occurs, the unavoidable reduction of xylem hydraulic conductance (Kstem) leads to partial or total stomatal closure and reduction of photosynthetic rates (Brodribb 2009). This is especially exacerbated during prolonged and very intense drought events (Brodribb and Holbrook 2006, McDowell 2011).
Plants are able to cope with embolism-induced loss of hydraulic conductance through (i) production of new xylem (e.g., Ameglio et al. 2002), (ii) repair of embolized xylem conduits (novel refilling, Salleo et al. 1996), and (iii) ion-mediated enhancement of residual stem hydraulic conductance (Kstem, the so-called ‘ionic effect’, Zwieniecki et al. 2001). While the production of new xylem conduits requires relatively long time intervals, refilling and ionic effects are potentially effective mechanisms for the short-term regulation of xylem water transport and, as a consequence, they could help plants face short-term drought stress.
Embolism reversal has been documented in >30 plant species (Brodersen and McElrone 2013) and, according to the most recent findings (Secchi and Zwieniechi 2012), the process is apparently based on an osmotic mechanism. Briefly, the currently accepted hypothesis proposes that the driving force to refill with water gas-filled conduits is generated by the input of solutes into the residual sap in the embolized vessel. The most reasonable osmotica involved are sugars such as sucrose obtained from starch depolymerization in xylem parenchyma cells and exported to conduits (e.g., Bucci et al. 2003, Salleo et al. 2006, Regier et al. 2009, Secchi and Zwieniechi 2011). However, the involvement of proteins and/or inorganic ions such as K+ cannot be excluded (Tyree et al. 1999, Neumann et al. 2010). Moreover, it is not clear whether phloem, whose integrity is apparently required for refilling to occur (Bucci et al. 2003, Salleo et al. 2004), is involved in the unloading of sugars and water into refilling conduits and/or in exportation of chemical signals for starch depolymerization (Nardini et al. 2011a).
The occurrence of an ion-mediated regulation of Kstem has also been documented in several angiosperm species (Nardini et al. 2011b). The ionic effect is supposed to be a consequence of the polyelectrolytic nature of pit membrane components (Zwieniecki et al. 2001, Gortan et al. 2011, Dusotoit-Coucaud et al. 2013). Recently, Lee et al. (2012) have suggested that the thickness, rather than porosity as originally hypothesized, of the bordered pit membrane changes in response to sap ion concentrations. From this view, ion-induced reduction of pit membrane thickness would be responsible for the decrease of hydraulic resistance of the pit water pathway. However, the exact mechanism underlying the ionic effect is still controversial (Van Doorn et al. 2011, Santiago et al. 2013). Positive correlations between anatomical features of pits and vessels, such as vessel grouping, and the ionic effect are in general agreement with the involvement of pit apertures (Jansen et al. 2011), but several questions are still unresolved. Most importantly, it is still unclear whether embolism repair and ionic effects are effectively adopted and coordinated by plants to alleviate xylem dysfunction under field conditions, although some recent studies have reported the occurrence of both phenomena in field-grown plants (Trifilò et al. 2003, 2008, 2011, Salleo et al. 2009).
On the basis of the above, it is reasonable to suppose that the ability of plants to recover from, or to compensate for, cavitation could improve their competitiveness and resistance to drought stress, especially in Mediterranean-type biomes where climate imposes high selective pressure for adequate leaf water supply (Nardini et al. 2014). However, clear-cut evidence supporting this hypothesis is still largely lacking. More information about the regulation of xylem water transport in Mediterranean plants is needed in order to predict the impact of increased summer drought frequency/intensity on vegetation in this biome (Martínez-Vilalta et al. 2002, Giorgi and Lionello 2008, Matusik et al. 2013). Hydraulic failure (i.e., plant desiccation due to inadequate water transport after cavitation) has been invoked as a possible causal factor of tree dieoff during drought events (McDowell et al. 2008, Hoffmann et al. 2011, Nardini et al. 2013). Therefore, a deeper understanding of species-specific regulation of xylem water transport could allow clarification of species-specific responses to aridity and prediction of responses of forest ecosystems to climate changes (Choat et al. 2012, Nardini et al. 2013, Sack and Scoffoni 2013).
This paper describes the diurnal and seasonal occurrence of embolism repair and ionic effects in three Mediterranean species (Ceratonia siliqua L., Olea europaea L. and Laurus nobilis L.) growing in the same habitat, with the aim of determining whether these mechanisms are employed by plants during periods of drought stress. Both laboratory and field measurements were performed, and the possible correlations between seasonal changes in ionic effects and xylem anatomical features as well as between embolism repair and starch availability were investigated.
Materials and methods
Experiments were performed in January (winter), May (spring), July (summer) and October (autumn) 2012 on 1-year (during January) to 2-year (in October) old stems collected from at least two adult specimens of three evergreen tree species growing on the campus of the Department of Biological Sciences and Environment, University of Messina, Italy (38° 15′ 41.34′′ N; 15° 35′ 51.67′′ ). The species investigated were C. siliqua L. (carob), O. europaea L. (olive) and L. nobilis L. (laurel). Carob and olive are typical components of dry sclerophyllous Mediterranean woodlands (Pignatti 1982), while L. nobilis is a sclerophyllous evergreen more restricted to mesic sites. Measurements were carried out at two different times of day: between 7:00 and 8:30 h (morning) on branches with low transpiration rates (<0.8 mmol m−2) and between 12:30 and 15:30 h (midday) on sun-exposed branches with high transpiration rates (see Table 2). During the entire experiment, trees were not irrigated and received only natural precipitation. Predawn leaf water potential (ψPD) under these conditions was measured on three basal covered leaves per species per season using a pressure chamber (3005 Plant Water Status Console, Soil Moisture Equipment Corp., Santa Barbara, CA, USA) (Table 1).
Means ± SD of photosynthetically active radiation (PAR), relative humidity (RH), air temperature (Tair) and predawn leaf water potential (ψPD) recorded in January, May, July and October 2012 at morning and midday (n = 12 for Tair, PAR and RH, n = 9 for ψPD). Different letters indicate significant differences for Tukey's pairwise comparisons (P < 0.01 for ψPD, P < 0.001 for all parameters).
| . | January . | May . | July . | October . | ||||
|---|---|---|---|---|---|---|---|---|
| Morning . | Midday . | Morning . | Midday . | Morning . | Midday . | Morning . | Midday . | |
| PAR (μmol m−2 s−1) | 35 ± 9a | 1000 ± 190b | 40 ± 10a | 1260 ± 286c | 50 ± 10a | 1570 ± 200d | 36 ± 8 | 1350 ± 160c |
| RH (%) | 60 ± 9abc | 55 ± 5a | 62 ± 8bc | 47 ± 2d | 65 ± 4c | 57 ± 2a | 65 ± 4b | 59 ± 4ab |
| Tair (°C) | 12 ± 2a | 16 ± 0.5b | 20 ± 0.5c | 26 ± 1.6d | 27 ± 1.7d | 32 ± 2e | 21 ± 2c | 25 ± 1.8d |
| ψPD (MPa) | −0.05 ± 0.02a | −0.08 ± 0.02bc | −0.1 ± 0.03b | −0.07 ± 0.02ac | ||||
| Rain (mm) | 195.8 | 102.4 | 120.6 | 323 | ||||
| . | January . | May . | July . | October . | ||||
|---|---|---|---|---|---|---|---|---|
| Morning . | Midday . | Morning . | Midday . | Morning . | Midday . | Morning . | Midday . | |
| PAR (μmol m−2 s−1) | 35 ± 9a | 1000 ± 190b | 40 ± 10a | 1260 ± 286c | 50 ± 10a | 1570 ± 200d | 36 ± 8 | 1350 ± 160c |
| RH (%) | 60 ± 9abc | 55 ± 5a | 62 ± 8bc | 47 ± 2d | 65 ± 4c | 57 ± 2a | 65 ± 4b | 59 ± 4ab |
| Tair (°C) | 12 ± 2a | 16 ± 0.5b | 20 ± 0.5c | 26 ± 1.6d | 27 ± 1.7d | 32 ± 2e | 21 ± 2c | 25 ± 1.8d |
| ψPD (MPa) | −0.05 ± 0.02a | −0.08 ± 0.02bc | −0.1 ± 0.03b | −0.07 ± 0.02ac | ||||
| Rain (mm) | 195.8 | 102.4 | 120.6 | 323 | ||||
Data for seasonal rain were recorded by the weather station of Torre Faro, Messina, Italy (http://www.torrefarometeo.altervista.org).
Means ± SD of photosynthetically active radiation (PAR), relative humidity (RH), air temperature (Tair) and predawn leaf water potential (ψPD) recorded in January, May, July and October 2012 at morning and midday (n = 12 for Tair, PAR and RH, n = 9 for ψPD). Different letters indicate significant differences for Tukey's pairwise comparisons (P < 0.01 for ψPD, P < 0.001 for all parameters).
| . | January . | May . | July . | October . | ||||
|---|---|---|---|---|---|---|---|---|
| Morning . | Midday . | Morning . | Midday . | Morning . | Midday . | Morning . | Midday . | |
| PAR (μmol m−2 s−1) | 35 ± 9a | 1000 ± 190b | 40 ± 10a | 1260 ± 286c | 50 ± 10a | 1570 ± 200d | 36 ± 8 | 1350 ± 160c |
| RH (%) | 60 ± 9abc | 55 ± 5a | 62 ± 8bc | 47 ± 2d | 65 ± 4c | 57 ± 2a | 65 ± 4b | 59 ± 4ab |
| Tair (°C) | 12 ± 2a | 16 ± 0.5b | 20 ± 0.5c | 26 ± 1.6d | 27 ± 1.7d | 32 ± 2e | 21 ± 2c | 25 ± 1.8d |
| ψPD (MPa) | −0.05 ± 0.02a | −0.08 ± 0.02bc | −0.1 ± 0.03b | −0.07 ± 0.02ac | ||||
| Rain (mm) | 195.8 | 102.4 | 120.6 | 323 | ||||
| . | January . | May . | July . | October . | ||||
|---|---|---|---|---|---|---|---|---|
| Morning . | Midday . | Morning . | Midday . | Morning . | Midday . | Morning . | Midday . | |
| PAR (μmol m−2 s−1) | 35 ± 9a | 1000 ± 190b | 40 ± 10a | 1260 ± 286c | 50 ± 10a | 1570 ± 200d | 36 ± 8 | 1350 ± 160c |
| RH (%) | 60 ± 9abc | 55 ± 5a | 62 ± 8bc | 47 ± 2d | 65 ± 4c | 57 ± 2a | 65 ± 4b | 59 ± 4ab |
| Tair (°C) | 12 ± 2a | 16 ± 0.5b | 20 ± 0.5c | 26 ± 1.6d | 27 ± 1.7d | 32 ± 2e | 21 ± 2c | 25 ± 1.8d |
| ψPD (MPa) | −0.05 ± 0.02a | −0.08 ± 0.02bc | −0.1 ± 0.03b | −0.07 ± 0.02ac | ||||
| Rain (mm) | 195.8 | 102.4 | 120.6 | 323 | ||||
Data for seasonal rain were recorded by the weather station of Torre Faro, Messina, Italy (http://www.torrefarometeo.altervista.org).
Means ± SD (n = 8) of gL, EL, ψL and pressure drops between the stem base and apex (ψxbasal – ψxapical) of morning and midday leaves of C. siliqua, O. europaea and L. nobilis recorded in January, May, July and October 2012.
| . | January . | May . | July . | October . | ||||
|---|---|---|---|---|---|---|---|---|
| Morning . | Midday . | Morning . | Midday . | Morning . | Midday . | Morning . | Midday . | |
| C. siliqua | ||||||||
| gL (mmol m−2 s−1) | 65 ± 9a | 245 ± 24b | 59 ± 8a | 251 ± 74b | 83 ± 7a | 275 ± 75b | 57 ± 7a | 250 ± 70b |
| EL (mmol m−2 s−1) | 0.8 ± 0.3a | 1.9 ± 0.3b | 0.6 ± 0.2a | 3.9 ± 1.2c | 0.8 ± 0.2a | 4.9 ± 1.5c | 0.7 ± 0.2a | 4 ± 1c |
| ψL (MPa) | −0.78 ± 0.04 | −99 ± 0.09b | −0.72 ± 0.09 | −1.23 ± 0.12c | −0.77 ± 0.1a | −1.73 ± 0.18d | −0.85 ± 0.06a | −1.4 ± 0.02e |
| (ψxbasal − ψxapical) (MPa) | 0.04 ± 0.01a | 0.08 ± 0.02b | 0.04 ± 0.01a | 0.13 ± 0.03c | 0.04 ± 0.01a | 0.18 ± 0.03d | 0.05 ± 0.02ab | 0.2 ± 0.03d |
| O. europaea | ||||||||
| gL (mmol m−2 s−1) | 70 ± 7a | 213 ± 40b | 61 ± 7a | 148 ± 44c | 78 ± 10a | 128 ± 23c | 76 ± 8a | 204 ± 54b |
| EL (mmol m−2 s−1) | 0.55 ± 0.1a | 1.49 ± 0.36b | 0.55 ± 0.03a | 2.48 ± 0.6c | 0.7 ± 0.1a | 4.1 ± 1.3c | 0.6 ± 0.01 | 3.5 ± 0.86c |
| ψL (MPa) | −0.89 ± 0.1a | −1.22 ± 0.09ad | −1.05 ± 0.07a | −1.86 ± 0.45b | −2.2 ± 0.2be | −2.9 ± 0.36c | −1.4 ± 0.1d | −2.4 ± 0.34e |
| (ψxbasal − ψxapical) (MPa) | 0.08 ± 0.02ac | 0.13 ± 0.03b | 0.04 ± 0.01a | 0.11 ± 0.03bc | 0.07 ± 0.03ac | 0.24 ± 0.04d | 0.06 ± 0.02a | 0.21 ± 0.03d |
| L. nobilis | ||||||||
| gL (mmol m−2 s−1) | 48 ± 10a | 158 ± 32b | 41 ± 12a | 138 ± 22b | 50 ± 15a | 100 ± 18c | 47.3 ± 11a | 130 ± 15b |
| EL (mmol m−2 s−1) | 0.44 ± 0.07a | 1.58 ± 0.6b | 0.7 ± 0.2a | 2.79 ± 0.5c | 0.65 ± 0.2a | 3.99 ± 1.4c | 0.4 ± 0.2a | 3.1 ± 0.8c |
| ψL (MPa) | −0.5 ± 0.06a | −1.03 ± 0.07b | −0.49 ± 0.1a | −1.58 ± 0.4c | −1.43 ± 0.5 c | −1.87 ± 0.17d | −0.92 ± 0.3b | −1.9 ± 0.2d |
| (ψxbasal − ψxapical) (MPa) | 0.06 ± 0.01a | 0.1 ± 0.03bc | 0.04 ± 0.01a | 0.12 ± 0.04b | 0.08 ± 0.02ac | 0.22 ± 0.02d | 0.05 ± 0.02a | 0.2 ± 0.02d |
| . | January . | May . | July . | October . | ||||
|---|---|---|---|---|---|---|---|---|
| Morning . | Midday . | Morning . | Midday . | Morning . | Midday . | Morning . | Midday . | |
| C. siliqua | ||||||||
| gL (mmol m−2 s−1) | 65 ± 9a | 245 ± 24b | 59 ± 8a | 251 ± 74b | 83 ± 7a | 275 ± 75b | 57 ± 7a | 250 ± 70b |
| EL (mmol m−2 s−1) | 0.8 ± 0.3a | 1.9 ± 0.3b | 0.6 ± 0.2a | 3.9 ± 1.2c | 0.8 ± 0.2a | 4.9 ± 1.5c | 0.7 ± 0.2a | 4 ± 1c |
| ψL (MPa) | −0.78 ± 0.04 | −99 ± 0.09b | −0.72 ± 0.09 | −1.23 ± 0.12c | −0.77 ± 0.1a | −1.73 ± 0.18d | −0.85 ± 0.06a | −1.4 ± 0.02e |
| (ψxbasal − ψxapical) (MPa) | 0.04 ± 0.01a | 0.08 ± 0.02b | 0.04 ± 0.01a | 0.13 ± 0.03c | 0.04 ± 0.01a | 0.18 ± 0.03d | 0.05 ± 0.02ab | 0.2 ± 0.03d |
| O. europaea | ||||||||
| gL (mmol m−2 s−1) | 70 ± 7a | 213 ± 40b | 61 ± 7a | 148 ± 44c | 78 ± 10a | 128 ± 23c | 76 ± 8a | 204 ± 54b |
| EL (mmol m−2 s−1) | 0.55 ± 0.1a | 1.49 ± 0.36b | 0.55 ± 0.03a | 2.48 ± 0.6c | 0.7 ± 0.1a | 4.1 ± 1.3c | 0.6 ± 0.01 | 3.5 ± 0.86c |
| ψL (MPa) | −0.89 ± 0.1a | −1.22 ± 0.09ad | −1.05 ± 0.07a | −1.86 ± 0.45b | −2.2 ± 0.2be | −2.9 ± 0.36c | −1.4 ± 0.1d | −2.4 ± 0.34e |
| (ψxbasal − ψxapical) (MPa) | 0.08 ± 0.02ac | 0.13 ± 0.03b | 0.04 ± 0.01a | 0.11 ± 0.03bc | 0.07 ± 0.03ac | 0.24 ± 0.04d | 0.06 ± 0.02a | 0.21 ± 0.03d |
| L. nobilis | ||||||||
| gL (mmol m−2 s−1) | 48 ± 10a | 158 ± 32b | 41 ± 12a | 138 ± 22b | 50 ± 15a | 100 ± 18c | 47.3 ± 11a | 130 ± 15b |
| EL (mmol m−2 s−1) | 0.44 ± 0.07a | 1.58 ± 0.6b | 0.7 ± 0.2a | 2.79 ± 0.5c | 0.65 ± 0.2a | 3.99 ± 1.4c | 0.4 ± 0.2a | 3.1 ± 0.8c |
| ψL (MPa) | −0.5 ± 0.06a | −1.03 ± 0.07b | −0.49 ± 0.1a | −1.58 ± 0.4c | −1.43 ± 0.5 c | −1.87 ± 0.17d | −0.92 ± 0.3b | −1.9 ± 0.2d |
| (ψxbasal − ψxapical) (MPa) | 0.06 ± 0.01a | 0.1 ± 0.03bc | 0.04 ± 0.01a | 0.12 ± 0.04b | 0.08 ± 0.02ac | 0.22 ± 0.02d | 0.05 ± 0.02a | 0.2 ± 0.02d |
Different letters indicate significant differences for Tukey's pairwise comparisons (P < 0.001).
Means ± SD (n = 8) of gL, EL, ψL and pressure drops between the stem base and apex (ψxbasal – ψxapical) of morning and midday leaves of C. siliqua, O. europaea and L. nobilis recorded in January, May, July and October 2012.
| . | January . | May . | July . | October . | ||||
|---|---|---|---|---|---|---|---|---|
| Morning . | Midday . | Morning . | Midday . | Morning . | Midday . | Morning . | Midday . | |
| C. siliqua | ||||||||
| gL (mmol m−2 s−1) | 65 ± 9a | 245 ± 24b | 59 ± 8a | 251 ± 74b | 83 ± 7a | 275 ± 75b | 57 ± 7a | 250 ± 70b |
| EL (mmol m−2 s−1) | 0.8 ± 0.3a | 1.9 ± 0.3b | 0.6 ± 0.2a | 3.9 ± 1.2c | 0.8 ± 0.2a | 4.9 ± 1.5c | 0.7 ± 0.2a | 4 ± 1c |
| ψL (MPa) | −0.78 ± 0.04 | −99 ± 0.09b | −0.72 ± 0.09 | −1.23 ± 0.12c | −0.77 ± 0.1a | −1.73 ± 0.18d | −0.85 ± 0.06a | −1.4 ± 0.02e |
| (ψxbasal − ψxapical) (MPa) | 0.04 ± 0.01a | 0.08 ± 0.02b | 0.04 ± 0.01a | 0.13 ± 0.03c | 0.04 ± 0.01a | 0.18 ± 0.03d | 0.05 ± 0.02ab | 0.2 ± 0.03d |
| O. europaea | ||||||||
| gL (mmol m−2 s−1) | 70 ± 7a | 213 ± 40b | 61 ± 7a | 148 ± 44c | 78 ± 10a | 128 ± 23c | 76 ± 8a | 204 ± 54b |
| EL (mmol m−2 s−1) | 0.55 ± 0.1a | 1.49 ± 0.36b | 0.55 ± 0.03a | 2.48 ± 0.6c | 0.7 ± 0.1a | 4.1 ± 1.3c | 0.6 ± 0.01 | 3.5 ± 0.86c |
| ψL (MPa) | −0.89 ± 0.1a | −1.22 ± 0.09ad | −1.05 ± 0.07a | −1.86 ± 0.45b | −2.2 ± 0.2be | −2.9 ± 0.36c | −1.4 ± 0.1d | −2.4 ± 0.34e |
| (ψxbasal − ψxapical) (MPa) | 0.08 ± 0.02ac | 0.13 ± 0.03b | 0.04 ± 0.01a | 0.11 ± 0.03bc | 0.07 ± 0.03ac | 0.24 ± 0.04d | 0.06 ± 0.02a | 0.21 ± 0.03d |
| L. nobilis | ||||||||
| gL (mmol m−2 s−1) | 48 ± 10a | 158 ± 32b | 41 ± 12a | 138 ± 22b | 50 ± 15a | 100 ± 18c | 47.3 ± 11a | 130 ± 15b |
| EL (mmol m−2 s−1) | 0.44 ± 0.07a | 1.58 ± 0.6b | 0.7 ± 0.2a | 2.79 ± 0.5c | 0.65 ± 0.2a | 3.99 ± 1.4c | 0.4 ± 0.2a | 3.1 ± 0.8c |
| ψL (MPa) | −0.5 ± 0.06a | −1.03 ± 0.07b | −0.49 ± 0.1a | −1.58 ± 0.4c | −1.43 ± 0.5 c | −1.87 ± 0.17d | −0.92 ± 0.3b | −1.9 ± 0.2d |
| (ψxbasal − ψxapical) (MPa) | 0.06 ± 0.01a | 0.1 ± 0.03bc | 0.04 ± 0.01a | 0.12 ± 0.04b | 0.08 ± 0.02ac | 0.22 ± 0.02d | 0.05 ± 0.02a | 0.2 ± 0.02d |
| . | January . | May . | July . | October . | ||||
|---|---|---|---|---|---|---|---|---|
| Morning . | Midday . | Morning . | Midday . | Morning . | Midday . | Morning . | Midday . | |
| C. siliqua | ||||||||
| gL (mmol m−2 s−1) | 65 ± 9a | 245 ± 24b | 59 ± 8a | 251 ± 74b | 83 ± 7a | 275 ± 75b | 57 ± 7a | 250 ± 70b |
| EL (mmol m−2 s−1) | 0.8 ± 0.3a | 1.9 ± 0.3b | 0.6 ± 0.2a | 3.9 ± 1.2c | 0.8 ± 0.2a | 4.9 ± 1.5c | 0.7 ± 0.2a | 4 ± 1c |
| ψL (MPa) | −0.78 ± 0.04 | −99 ± 0.09b | −0.72 ± 0.09 | −1.23 ± 0.12c | −0.77 ± 0.1a | −1.73 ± 0.18d | −0.85 ± 0.06a | −1.4 ± 0.02e |
| (ψxbasal − ψxapical) (MPa) | 0.04 ± 0.01a | 0.08 ± 0.02b | 0.04 ± 0.01a | 0.13 ± 0.03c | 0.04 ± 0.01a | 0.18 ± 0.03d | 0.05 ± 0.02ab | 0.2 ± 0.03d |
| O. europaea | ||||||||
| gL (mmol m−2 s−1) | 70 ± 7a | 213 ± 40b | 61 ± 7a | 148 ± 44c | 78 ± 10a | 128 ± 23c | 76 ± 8a | 204 ± 54b |
| EL (mmol m−2 s−1) | 0.55 ± 0.1a | 1.49 ± 0.36b | 0.55 ± 0.03a | 2.48 ± 0.6c | 0.7 ± 0.1a | 4.1 ± 1.3c | 0.6 ± 0.01 | 3.5 ± 0.86c |
| ψL (MPa) | −0.89 ± 0.1a | −1.22 ± 0.09ad | −1.05 ± 0.07a | −1.86 ± 0.45b | −2.2 ± 0.2be | −2.9 ± 0.36c | −1.4 ± 0.1d | −2.4 ± 0.34e |
| (ψxbasal − ψxapical) (MPa) | 0.08 ± 0.02ac | 0.13 ± 0.03b | 0.04 ± 0.01a | 0.11 ± 0.03bc | 0.07 ± 0.03ac | 0.24 ± 0.04d | 0.06 ± 0.02a | 0.21 ± 0.03d |
| L. nobilis | ||||||||
| gL (mmol m−2 s−1) | 48 ± 10a | 158 ± 32b | 41 ± 12a | 138 ± 22b | 50 ± 15a | 100 ± 18c | 47.3 ± 11a | 130 ± 15b |
| EL (mmol m−2 s−1) | 0.44 ± 0.07a | 1.58 ± 0.6b | 0.7 ± 0.2a | 2.79 ± 0.5c | 0.65 ± 0.2a | 3.99 ± 1.4c | 0.4 ± 0.2a | 3.1 ± 0.8c |
| ψL (MPa) | −0.5 ± 0.06a | −1.03 ± 0.07b | −0.49 ± 0.1a | −1.58 ± 0.4c | −1.43 ± 0.5 c | −1.87 ± 0.17d | −0.92 ± 0.3b | −1.9 ± 0.2d |
| (ψxbasal − ψxapical) (MPa) | 0.06 ± 0.01a | 0.1 ± 0.03bc | 0.04 ± 0.01a | 0.12 ± 0.04b | 0.08 ± 0.02ac | 0.22 ± 0.02d | 0.05 ± 0.02a | 0.2 ± 0.02d |
Different letters indicate significant differences for Tukey's pairwise comparisons (P < 0.001).
Measuring gas exchange and leaf water potential
Leaf conductance to water vapour (gL), transpiration rate (EL) and leaf water potential (ψL) were measured on a diurnal/seasonal scale on at least eight leaves per species using a portable steady-state porometer (LI 1600, LiCor Inc., Lincoln, NE, USA) and the pressure chamber (see above). Air temperature (Tair), air relative humidity (RH) and photosynthetically active radiation (PAR) were also recorded using the sensors of the porometer cuvette.
Measuring xylem sap potassium concentration
In order to estimate diurnal and seasonal changes in xylem sap potassium concentration ([K+]), 80- to 120-cm-long shoots were collected, immediately defoliated and transported to the laboratory. Xylem sap was extracted using the vacuum chamber technique (Améglio et al. 2004). The cut surface of the shoot was rinsed with deionized water to remove phloem sap residues and debris and put into a 1.5-ml Eppendorf vial, which rested in a beaker filled with ice to minimize evaporation during sap collection. Potassium concentration was then measured with a [K+]-selective electrode (Cardy Compact Ion Meter, Model C-131, Horiba Ltd, Kyoto, Japan).
Measuring ion-mediated changes in hydraulic conductance
This experiment was designed to assess the magnitude of the ionic effect and its eventual changes on a seasonal basis. Ion-mediated increases in stem hydraulic conductance (ΔKstem) were tested in fully hydrated samples by perfusing samples with KCl solutions at increasing concentrations (10, 25 and 50 mM). The Kstem measured under KCl perfusion was compared with values recorded under perfusion with a reference solution (commercial mineral water containing 0.51 mM Ca2+, 0.07 mM Mg2+, 0.08 mM Na+, 0.03 mM NO3−, 0.96 mM HCO3−, 0.15 mM SO42− and 0.01 mM F−, with [K+] adjusted to 3 mM). This reference perfusion fluid was selected to prevent spurious hydraulic effects caused by the use of deionized water (Nardini et al. 2007). Stem samples were cut off under distilled water and immediately transported to the laboratory where they were re-cut to a length of 18 cm and connected to a hydraulic apparatus (Xyl'Em, Xylem Embolism Meter, Bronkhorst, Montigny les Cormeilles, France). This stem length was selected on the basis of the species-specific vessel length (see below), in order to minimize the number of conduits cut open at both ends (Gascò et al. 2006). Samples were first flushed with the reference solution at P = 0.2 MPa for 20 min to remove native embolism. The Kstem was recorded at P = 7.5 kPa with reference fluid and then with KCl solutions at increasing concentrations. At each concentration level, measurements were continued until the flow became stable (∼10 and 30 min for the reference and KCl solutions, respectively). At least five stems per species and per month were tested.
In order to evaluate the actual occurrence of ion-mediated Kstem increase in planta, as well as its impact on plant water transport efficiency, measurements of ΔKstem were performed on samples collected in the field both in the morning and at midday on a seasonal scale. ΔKstem of samples with native embolism levels were thus obtained comparing Kstem measured with a perfused solution with [K+] equal to the concentration recorded in planta (at the same time of day and month in which samples were collected) with values measured by perfusing the reference solution. Measurements were performed on the same samples used to measure the loss of stem hydraulic conductance (see below).
It should be noted that data gathered on fully hydrated samples represent a potential increase of Kstem in response to potassium ions. By contrast, data gathered on samples with different degrees of native embolism provide information about the actual enhancement of Kstem associated with the ionic effect in planta, taking into account both the degree of xylem dysfunction (see Gascò et al. 2006) and xylem sap [K+].
Measuring percentage loss of stem hydraulic conductance
On the basis of (i) the different [K+] recorded at midday versus morning and (ii) the seasonal changes in the magnitude of the ionic effect (see the Results section), an experiment was designed to estimate the actual buffering effect of the ion-mediated enhancement of residual Kstem on the overall loss of stem hydraulic conductance, on both a diurnal and a seasonal scale. Daily and seasonal changes of ‘reference’ percentage loss of stem hydraulic conductance (PLC) values were also estimated with the reference solution in order to check the ability of stems to refill embolized xylem conduits.
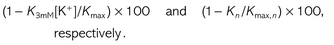
All hydraulic measurements were performed at a temperature of 20 °C.
Xylem anatomy
To select the length of stem samples to be used for hydraulic measurements (see above), the maximum xylem conduit lengths of four stems per species were determined using the silicone-injection technique (Sperry et al. 2005). Samples were connected to the hydraulic apparatus, flushed with water at P = 0.2 MPa to remove native embolism and then injected at P = 0.5 MPa for 3 h with silicone (Rhodorsil RTV-141, Rhodia, Cranbury, NJ, USA) mixed with a blue pigment (Pentasol, Prochima, Pesaro, Italy). After ∼12 h (i.e., the time needed for silicone hardening) stems were progressively cut into 2-cm-long segments. Conduits filled with blue silicone and the total number of conduits were counted under a microscope on sequential cross-sections, and the conduit length distribution was calculated as proposed by Sperry et al. (2005). The length of stems containing at least 60% of intact conduits was 14 cm for carob and olive and 12 cm for laurel.
The stems used for PLC measurements were cross-sectioned for anatomical analysis. Sections 30 µm thick were obtained with a microtome and briefly rinsed with distilled water before immersion for 1 min in a Lugol solution (iodine–potassium iodide) (Salleo et al. 2004, 2009). After rinsing samples again to remove excess stain, sections were observed under a microscope (Laborlux S, Leitz Esselte, Leitz GmbH, Stuttgart, Germany) equipped with a digital camera (Leica DC300F, Leica Camera AG, Solms, Germany) connected to a computer. For each section, eight to 15 different microscopic fields, together covering at least 60% of the section, were observed at ×25 magnification. The solitary vessel index, VS (ratio of total number of solitary vessels to total vessel groupings), and the vessel multiple fraction, FVM (ratio of grouped vessels to total number of vessels), were calculated (Jansen et al. 2011). Moreover, the percentage of xylem parenchyma cells with ‘high starch content’ (HSC-cells, i.e., starch granules filled >50% of the cell lumen) in relation to the total number of xylem parenchyma cells per cross-section was recorded (Salleo et al. 2004, 2009). This procedure allowed us to check whether seasonal changes of ionic effect and refilling were related to vessel grouping and starch content of xylem parenchyma cells, respectively.
Measurements of leaf specific stem hydraulic conductivity in planta
In order to get additional experimental support for laboratory measurements of Kstem, leaf specific stem hydraulic conductivity (LSCstem) was estimated in the field, both in the morning and at midday, using the evaporative flux method (EFM) (Nardini et al. 2010, Trifilò et al. 2013).
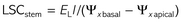
It has to be noted that LSCstem is not equivalent to values of Kstem because in the first the transpiration rate per unit leaf area of a single leaf was measured, while the values of stem hydraulic conductance take into account the actual and total flow through the stem xylem. However, estimating LSCstem in planta could give useful information about the actual impact of both refilling and ionic effect mechanisms on maintaining hydraulic function. In any case, data recorded with EFM must be interpreted with caution because of the intrinsic experimental limits of this methodology, i.e., the possible inaccurate estimates of branch-level transpiration rate and related water potential drop. For example, the boundary layer conductance in the chamber of the porometer was probably different from that experienced by bagged leaves used to estimate water potential gradient.
Statistics
Data were analysed with the SigmaStat 2.0 (SPSS, Inc., Chicago, IL, USA) statistics package. Differences on seasonal and daily scales in measured parameters and between treatments were tested for each species using one-way ANOVA and Tukey's tests.
Results
Environmental and water relations data
During measurements, the PAR was ∼40 µmol m−2 s−1 in the morning, and ranged between 1000 (January) and 1600 µmol m−2 s−1 (July) at midday (Table 1). Air humidity was higher in the morning whereas Tair reached maximum values (∼30 °C) during July at midday.
Table 2 shows the gL, EL and ψL values recorded in the morning and at midday in the three study species. In C. siliqua, gL ranged between 60 and 250 mmol m−2 s−1 in the morning and at midday, respectively. The most negative ψL values were recorded in July at midday (about −1.73 MPa), while the morning values were higher and not different throughout the year (about −0.8 MPa). In olive, gL was always ∼70 mmol m−2 s−1 in the morning versus ∼200 mmol m−2 s−1 during January and October at midday. In laurel, gL of the morning samples was ∼45 mmol m−2 s−1 throughout the year. At midday, gL was ∼ 140 mmol m−2 s−1 in January, May and October, while a statistically significant decrease was recorded in July (∼100 mmol m−2 s−1). In these two species, the lowest ψL values were recorded in July at midday (−2.9 ± 0.36 MPa in olive and −1.87 ± 0.17 MPa in laurel), despite the occurrence of partial stomatal closure.
In all the three species, the transpiration rates as well as the pressure drops between stem base and apex (ψxbasal – ψxapical) were larger at midday than in the early morning.
Xylem sap [K+]
Xylem sap [K+] changed on a diurnal and seasonal time scale in all the species studied (Figure 1). In carob, the early morning values of xylem sap [K+] ranged from ∼2 mM (in January) to ∼5 mM (in the other months). At midday, [K+] increased to ∼5 mM in January and 12 mM in October. In olive and laurel, the values of xylem sap [K+] were quite constant throughout the year in the morning hours (∼5 mM), while midday [K+] increased to 14 mM.
Mean values ± SD (n = 8) of xylem sap [K+] as measured in morning (black columns) and midday (white columns) branches of (a) C. siliqua, (b) O. europaea and (c) L. nobilis in 2012. Different letters indicate significant differences for Tukey's pairwise comparisons (P < 0.001).
Measurements of ion-mediated changes in Kstem
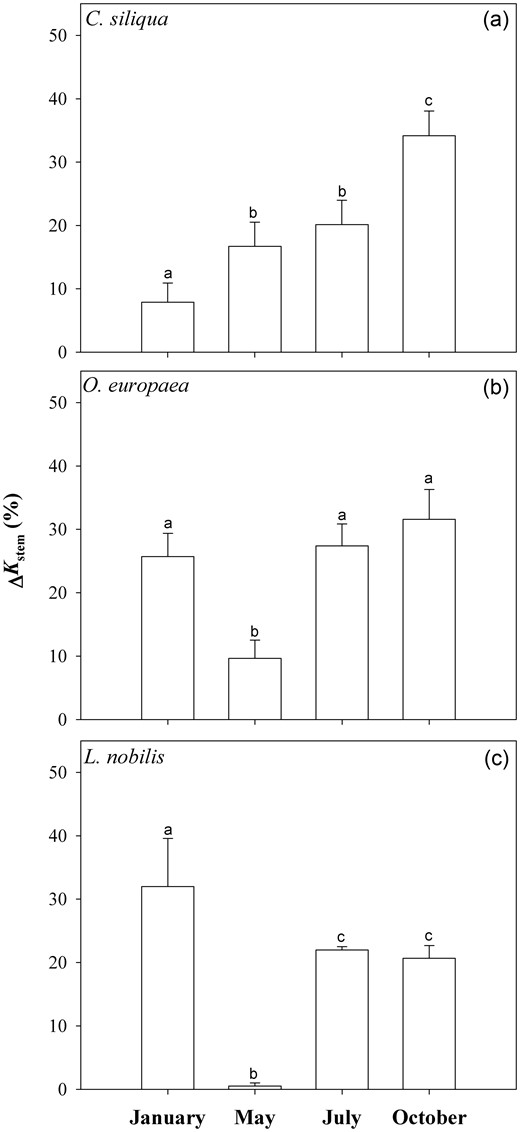
Fully hydrated samples of carob and olive trees showed the highest ΔKstem values when perfused with 25 mM KCl solution, while L. nobilis showed a further increase of Kstem to 50 mM KCl (data not shown). Hence, Figure 2 reports maximum ΔKstem values obtained on fully hydrated samples by perfusing solutions enriched with 25 mM KCl for carob and olive and 50 mM KCl for laurel. The magnitude of the ΔKstem differed in the three species, as in C. siliqua it was less than +10% in January, about +20% in May and July, and reached the maximum in October (about +34%) (Figure 2a). In O. europaea, the ionic effect was lowest in May (10%) and quite constant during the other months (about +30%) (Figure 2b). In L. nobilis, ΔKstem increased by 30% in January, but was significantly less in July and October (about +20%) while no ionic effect was recorded in May (Figure 2c).
Means ± SD (n = 5) of the percentage increase Q12of stem hydraulic conductance (ΔKstem) of fully hydrated samples as induced by 25 mM KCl in carob (a) and olive (b) or 50 mM KCl in laurel (c) compared with the reference solution. Different letters indicate significant differences for Tukey's pairwise comparisons (P < 0.001).
Different ion-mediated increases in stem hydraulic conductance were recorded in embolized samples when midday samples were compared with morning ones, using potassium concentrations mimicking values recorded in planta (Figure 3). In all the species, ΔKstem in the morning was always very low or zero, as a consequence of the low native [K+]. By contrast, ΔKstem increased significantly at midday in all seasons except in January for carob and in May for olive and laurel. It can be noted that ΔKstem of embolized samples at midday showed, on a seasonal basis, a trend quite similar to that recorded in fully hydrated stems, although with different and generally higher values. In particular, ΔKstem in carob was about +5% in January, +35% in May and July, and +80% in October (Figure 3a). In olive, values of about +50% were recorded in all study periods, except in May when ΔKstem was only about +18% (Figure 3b). In laurel, ΔKstem was as high as +45% in all months except in May (Figure 3c). It is worth noting that increases of Kstem to ∼80% (in carob) and ∼45–50% (in olive and laurel) were recorded in embolized samples by perfusing solution with only 15 mM KCl, corresponding to native potassium levels in the xylem sap. By contrast, values not much higher than about +35% were recorded in the same months in fully hydrated samples perfusing 25 mM (for carob and olive) or 50 mM (for laurel) KCl solutions (Figures 2 and 3).
Means ± SD (n = 8) of the percentage ΔKstem of embolized samples as induced by a reference solution enriched with [K+] similar to the concentration recorded in planta compared with the reference solution. Different letters indicate significant differences for Tukey's pairwise comparisons (P < 0.001). Grey and dashed grey columns refer to midday and morning samples, respectively.
Daily and seasonal PLC changes
The PLC of C. siliqua during perfusion with the reference solution did not differ between morning and midday in January (PLC = 25%) (Figure 4a). By contrast, in May and July PLC values as high as 55 and 40% were recorded in midday and morning samples, respectively. In October, an increase of PLC was observed both at midday and in the morning (80 and 55%, respectively). However, when stems were perfused with a solution with [K+] comparable to that recorded in planta (see Figure 1), the PLC of midday branches was significantly lower than that recorded at low potassium concentration. In fact, at native [K+] levels PLC was ∼40% in May and July and ∼60% in October. In other words, diurnal refilling was observed in C. siliqua, and the midday loss of hydraulic conductance was further buffered by the increase of [K+] in the xylem sap that enhanced residual Kstem.
Means ± SD (n = 8) of the PLC as measured in (a) C. siliqua, (b) O. europaea and (c) L. nobilis during 2012. Percentage loss of stem hydraulic conductance was obtained after perfusion with a solution containing 3 mM [K+] or after perfusion with a solution containing potassium concentrations corresponding to [K+] values as measured in planta (i.e., native [K+]). White and dashed white columns refer to midday samples, grey and dashed grey columns to morning samples. Different letters indicate significant differences for Tukey's pairwise comparisons (P < 0.001).
In O. europaea, diurnal embolism repair was observed in January, in July and, to a lesser extent, in October (Figure 4b). The PLC at 3 mM [K+] in midday branches was ∼55% in January, July and October, while in the morning it declined to ∼50% in January and ∼40% in July and October. When midday branches were perfused with the reference solution mimicking the sap [K+] recorded in planta, the PLC was significantly lower in all study periods except May. In laurel, diurnal changes of standard PLC were observed in all the 4 months (Figure 4c). Ion-mediated changes in stem hydraulic conductance allowed lower midday PLCn in January, July and October, but not in May.
Xylem anatomy
The starch content in stem parenchyma cells changed during the season in all three species (Table 3). In C. siliqua, the maximum percentage of HSC cells was observed in May (∼80%) and the minimum in July and October (∼4%). In each study period, however, morning and midday HSC percentages were not different, except in January. In olive, the percentage of HSC changed on a diurnal scale in January and July, when it was higher in the morning than at midday. A similar diurnal difference in the amount of HSC was observed in laurel in all the study periods with the only exception of January, when no changes in starch content were detected.
Means ± SD (n = 8) of the percentage of stem parenchyma cells with HSC relative to the total as recorded in the midday and morning branches of C. siliqua, O. europaea and L. nobilis in 2012.
| . | C. siliqua . | O. europaea . | L. nobilis . | |||
|---|---|---|---|---|---|---|
| Midday . | Morning . | Midday . | Morning . | Midday . | Morning . | |
| (%) . | (%) . | (%) . | ||||
| January | 27 ± 6a | 12 ± 5b | 20 ± 4a | 35 ± 5b | 15 ± 4a | 12 ± 4a |
| May | 79 ± 6c | 82 ± 5c | 73 ± 9c | 66 ± 11c | 14 ± 5a | 54 ± 6b |
| July | 4 ± 4b | 12 ± 5b | 34 ± 5b | 71 ± 10c | 3 ± 1c | 17 ± 3a |
| October | 5 ± 4b | 14 ± 6b | 21 ± 4ab | 26 ± 6ab | 28 ± 3d | 38 ± 4e |
| . | C. siliqua . | O. europaea . | L. nobilis . | |||
|---|---|---|---|---|---|---|
| Midday . | Morning . | Midday . | Morning . | Midday . | Morning . | |
| (%) . | (%) . | (%) . | ||||
| January | 27 ± 6a | 12 ± 5b | 20 ± 4a | 35 ± 5b | 15 ± 4a | 12 ± 4a |
| May | 79 ± 6c | 82 ± 5c | 73 ± 9c | 66 ± 11c | 14 ± 5a | 54 ± 6b |
| July | 4 ± 4b | 12 ± 5b | 34 ± 5b | 71 ± 10c | 3 ± 1c | 17 ± 3a |
| October | 5 ± 4b | 14 ± 6b | 21 ± 4ab | 26 ± 6ab | 28 ± 3d | 38 ± 4e |
Different letters indicate significant differences for Tukey's pairwise comparisons (P < 0.001).
Means ± SD (n = 8) of the percentage of stem parenchyma cells with HSC relative to the total as recorded in the midday and morning branches of C. siliqua, O. europaea and L. nobilis in 2012.
| . | C. siliqua . | O. europaea . | L. nobilis . | |||
|---|---|---|---|---|---|---|
| Midday . | Morning . | Midday . | Morning . | Midday . | Morning . | |
| (%) . | (%) . | (%) . | ||||
| January | 27 ± 6a | 12 ± 5b | 20 ± 4a | 35 ± 5b | 15 ± 4a | 12 ± 4a |
| May | 79 ± 6c | 82 ± 5c | 73 ± 9c | 66 ± 11c | 14 ± 5a | 54 ± 6b |
| July | 4 ± 4b | 12 ± 5b | 34 ± 5b | 71 ± 10c | 3 ± 1c | 17 ± 3a |
| October | 5 ± 4b | 14 ± 6b | 21 ± 4ab | 26 ± 6ab | 28 ± 3d | 38 ± 4e |
| . | C. siliqua . | O. europaea . | L. nobilis . | |||
|---|---|---|---|---|---|---|
| Midday . | Morning . | Midday . | Morning . | Midday . | Morning . | |
| (%) . | (%) . | (%) . | ||||
| January | 27 ± 6a | 12 ± 5b | 20 ± 4a | 35 ± 5b | 15 ± 4a | 12 ± 4a |
| May | 79 ± 6c | 82 ± 5c | 73 ± 9c | 66 ± 11c | 14 ± 5a | 54 ± 6b |
| July | 4 ± 4b | 12 ± 5b | 34 ± 5b | 71 ± 10c | 3 ± 1c | 17 ± 3a |
| October | 5 ± 4b | 14 ± 6b | 21 ± 4ab | 26 ± 6ab | 28 ± 3d | 38 ± 4e |
Different letters indicate significant differences for Tukey's pairwise comparisons (P < 0.001).
All species showed a significantly higher value of the VS in May (Table 4), when it was 0.57 ± 0.07 (C. siliqua), 0.56 ± 0.04 (O. europaea) and 0.49 ± 0.06 (L. nobilis). A different behaviour was recorded for FVM of the three species. Vessel multiple fraction of carob was highest in July and October (∼0.30). In olive, FVM peaked in January (0.35 ± 0.03), while laurel showed the highest values in January, July and October (∼0.30).
Means ± SD (n = 8) of the FVM and VS in branchwood of C. siliqua, O. europaea and L. nobilis in 2012.
| . | C. siliqua . | O. europaea . | L. nobilis . | |||
|---|---|---|---|---|---|---|
| FVM . | VS . | FVM . | VS . | FVM . | VS . | |
| January | 0.25 ± 0.01a | 0.49 ± 0.02a | 0.35 ± 0.03a | 0.49 ± 0.05a | 0.31 ± 0.03a | 0.39 ± 0.06a |
| May | 0.25 ± 0.03a | 0.57 ± 0.07b | 0.23 ± 0.03b | 0.56 ± 0.04b | 0.25 ± 0.02b | 0.49 ± 0.06b |
| July | 0.30 ± 0.03b | 0.46 ± 0.09a | 0.28 ± 0.02c | 0.50 ± 0.04a | 0.32 ± 0.02a | 0.34 ± 0.07a |
| October | 0.29 ± 0.03b | 0.50 ± 0.06a | 0.30 ± 0.03c | 0.48 ± 0.04a | 0.29 ± 0.05ab | 0.43 ± 0.05ab |
| P-value | <0.050 | <0.050 | <0.001 | <0.01 | <0.001 | <0.001 |
| . | C. siliqua . | O. europaea . | L. nobilis . | |||
|---|---|---|---|---|---|---|
| FVM . | VS . | FVM . | VS . | FVM . | VS . | |
| January | 0.25 ± 0.01a | 0.49 ± 0.02a | 0.35 ± 0.03a | 0.49 ± 0.05a | 0.31 ± 0.03a | 0.39 ± 0.06a |
| May | 0.25 ± 0.03a | 0.57 ± 0.07b | 0.23 ± 0.03b | 0.56 ± 0.04b | 0.25 ± 0.02b | 0.49 ± 0.06b |
| July | 0.30 ± 0.03b | 0.46 ± 0.09a | 0.28 ± 0.02c | 0.50 ± 0.04a | 0.32 ± 0.02a | 0.34 ± 0.07a |
| October | 0.29 ± 0.03b | 0.50 ± 0.06a | 0.30 ± 0.03c | 0.48 ± 0.04a | 0.29 ± 0.05ab | 0.43 ± 0.05ab |
| P-value | <0.050 | <0.050 | <0.001 | <0.01 | <0.001 | <0.001 |
Different letters indicate significant differences for Tukey's pairwise comparisons. The P values are also given.
Means ± SD (n = 8) of the FVM and VS in branchwood of C. siliqua, O. europaea and L. nobilis in 2012.
| . | C. siliqua . | O. europaea . | L. nobilis . | |||
|---|---|---|---|---|---|---|
| FVM . | VS . | FVM . | VS . | FVM . | VS . | |
| January | 0.25 ± 0.01a | 0.49 ± 0.02a | 0.35 ± 0.03a | 0.49 ± 0.05a | 0.31 ± 0.03a | 0.39 ± 0.06a |
| May | 0.25 ± 0.03a | 0.57 ± 0.07b | 0.23 ± 0.03b | 0.56 ± 0.04b | 0.25 ± 0.02b | 0.49 ± 0.06b |
| July | 0.30 ± 0.03b | 0.46 ± 0.09a | 0.28 ± 0.02c | 0.50 ± 0.04a | 0.32 ± 0.02a | 0.34 ± 0.07a |
| October | 0.29 ± 0.03b | 0.50 ± 0.06a | 0.30 ± 0.03c | 0.48 ± 0.04a | 0.29 ± 0.05ab | 0.43 ± 0.05ab |
| P-value | <0.050 | <0.050 | <0.001 | <0.01 | <0.001 | <0.001 |
| . | C. siliqua . | O. europaea . | L. nobilis . | |||
|---|---|---|---|---|---|---|
| FVM . | VS . | FVM . | VS . | FVM . | VS . | |
| January | 0.25 ± 0.01a | 0.49 ± 0.02a | 0.35 ± 0.03a | 0.49 ± 0.05a | 0.31 ± 0.03a | 0.39 ± 0.06a |
| May | 0.25 ± 0.03a | 0.57 ± 0.07b | 0.23 ± 0.03b | 0.56 ± 0.04b | 0.25 ± 0.02b | 0.49 ± 0.06b |
| July | 0.30 ± 0.03b | 0.46 ± 0.09a | 0.28 ± 0.02c | 0.50 ± 0.04a | 0.32 ± 0.02a | 0.34 ± 0.07a |
| October | 0.29 ± 0.03b | 0.50 ± 0.06a | 0.30 ± 0.03c | 0.48 ± 0.04a | 0.29 ± 0.05ab | 0.43 ± 0.05ab |
| P-value | <0.050 | <0.050 | <0.001 | <0.01 | <0.001 | <0.001 |
Different letters indicate significant differences for Tukey's pairwise comparisons. The P values are also given.
When midday values of ΔKstem were plotted versus corresponding PLC at 3 mM [K+] as recorded in all species on a seasonal basis, a positive correlation emerged between the two parameters (Figure 5a). Moreover, ΔKstem was significantly correlated to FVM (Figure 5b). Therefore, ion-mediated changes of stem hydraulic conductance increased as a function of both higher xylem dysfunction and higher vessel grouping. No correlation was found between the diurnal change in stem starch content (i.e., HSC of midday branches − HSC of morning branches) and the daily change of PLC (data not shown).
(a) Means ± SD of ΔKstem (%) recorded in fully hydrated samples as a function of means ± SD PLC at 3 mM [K+] for the three species; (b) means ± SD of ΔKstem (%) recorded in fully hydrated samples as a function of means ± SD of FVM for the three species. The regression line, R2 and P values are reported.
Field measurements of LSCstem
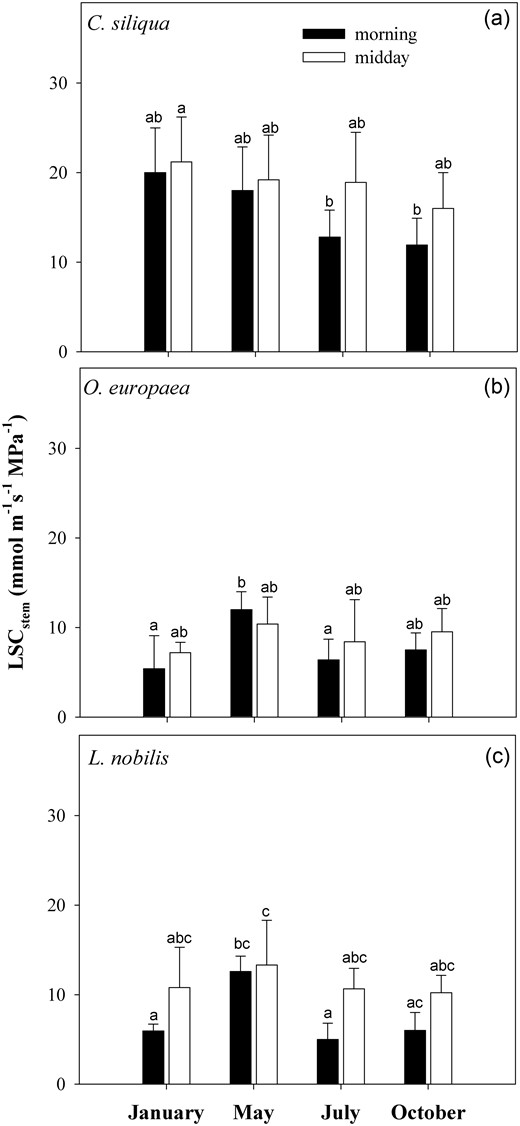
Figure 6 reports values of LSCstem as measured in planta using the EFM technique. In all the three species, no statistically significant differences were recorded in morning versus midday samples throughout the year. In C. siliqua, values of LSCstem of ∼20 mmol m−1 s−1 MPa−1 were recorded except in the early morning samples in July and October when LSCstem was <12 mmol m−1 s−1 MPa−1 (Figure 6a). In olive and laurel samples, LSCstem ranged from ∼5 mmol m−1 s−1 MPa−1 to ∼12 mmol m−1 s−1 MPa−1 throughout the year (Figure 6b and c).
Means ± SD (n = 6) of LSCstem as measured in planta using the EFM in morning (black columns) and in midday (white columns) branches of (a) C. siliqua, (b) O. europaea and (c) L. nobilis in 2012. Different letters indicate significant differences for Tukey's pairwise comparisons (P < 0.001).
Discussion
The main goal of the present study was to assess whether Mediterranean plants adopt ion-mediated regulation of xylem water transport as well as embolism repair processes to cope with seasonal drought stress. Our data suggest that the drought responses of the species studied were indeed based on the fine regulation of long-distance water transport, obtained by both diurnal and seasonal xylem refilling patterns, as well as by transient ion-mediated enhancement of Kstem.
Ionic effect
Changes in the magnitude of the ionic effect as well as in xylem sap [K+] were recorded in all three species on a seasonal scale (Figures 1–3). As a consequence of the increase of [K+] on a diurnal basis (Figure 1), Kstem was significantly enhanced at midday, especially in the months in which the xylem sensitivity to ions was higher (October for carob, January, June and October for olive, and January for laurel). As a consequence, native PLC measured with solutions mimicking the actual sap potassium content was found to be substantially different from PLC standard values recorded with a reference low-[K+] solution (Figure 4). In particular, when the ionic effect was present and [K+] was high, the PLCn was lower than the reference PLC. Nevertheless, these data and the higher ΔKstem values recorded in embolized stems than in fully hydrated samples (Figures 2 and 3) indicate that the magnitude of ion-mediated enhancement of Kstem is itself a function of PLC, as already reported in previous studies (Gascò et al. 2006, Trifilò et al. 2008). The positive correlation recorded between ΔKstem and PLC values (Figure 5a) clearly confirms this view and suggests that the ionic effect is an effective mechanism utilized by plants to alleviate the impact of xylem embolism, as previously hypothesized but only partially demonstrated in planta (Gascò et al. 2007, Trifilò et al. 2008, 2011). Our data also suggest that drought-stressed plants enrich their xylem sap with potassium ions to enhance the magnitude of the ionic effect, especially when embolism-induced xylem dysfunction develops in their transport systems. In fact, the highest sap [K+] (Figure 1) was recorded in the months in which the ionic effect was greater and PLC was larger (Figures 2 and 4). In any case, the possible source of ions loaded into xylem sap remains unclear although the most likely candidate is the phloem (Zwieniecki et al. 2004, Metzner et al. 2010).
Data reported in the present study reinforce the notion that the relative magnitude of the ionic effect is related to vessel arrangement (Jansen et al. 2011), as both ΔKstem and sap ionic strength were found to be higher in the species/season when xylem conduits appeared highly grouped (Figure 5). Jansen et al. (2011) showed a highly significant correlation between the ionic effect and some vessel grouping parameters, especially the portion of vessel walls in contact with neighbouring vessels. The ionic effect is larger in xylem with highly grouped vessels because the fraction of intervessel contacts and related radial flows through pit membranes is correlated to vessel grouping. We did not quantify the anatomical and biochemical features of pit membranes, but our results reinforce the hypothesis that xylem anatomy, and in particular the extent of vessel-to-vessel connections, is directly involved in determining the ionic effect, at both inter- and intra-specific (i.e., seasonal) scales. Recent and classical studies have suggested that cambial activity and vessel arrangement in early versus late wood may be affected by different environmental events (e.g., fire, drought, salt stress, flooding and low temperature) (e.g., Madany et al. 1982, Lovisolo and Schubert 1998, St George et al. 2002, Schmitz et al. 2006, Eilmann et al. 2009, Trifilò et al. 2013). This apparently confers to plants a large flexibility in terms of xylem construction in response to micro- and macro-environmental changes (Fonti et al. 2010), thus making it possible to modulate water transport as a function of environmental factors (Nardini et al. 2012, Zwieniecki and Secchi 2012). Of course, this does not exclude the hypothesis that ion-mediated changes of Kstem can be modulated rapidly also via modifications of the biochemical features of pit membrane structure (Zwieniecki and Secchi 2012).
Refilling
All species under study showed the ability to reverse xylem embolism (Figure 4). In fact, when midday losses of stem hydraulic conductance were compared with morning PLC values as recorded with the same reference solution (i.e., PLC at 3 mM [K+]), changes in the amount of embolism became apparent in all three species. In particular, in carob trees the diurnal xylem refilling was evident in all months except January. In olive and laurel, refilling was not observed only in May (Figure 4b and c).
Taking into account that the xylem vessel diameter of all three species ranged between 15 and 80 µm (data not shown), and on the basis of Henry's law, it can be predicted that in the species under study a passive bubble collapse could have occurred in a range of pressure between −0.02 and −0.0018 MPa. Therefore, a passive refilling mechanism, especially in some months, could not be ruled out considering unusual high (i.e., not very negative) values of ψPD.
Previous studies have led to the hypothesis that refilling processes are driven by the depolymerization of starch (Bucci et al. 2003, Salleo et al. 2006, Secchi et al. 2011). This hypothesis is only partially confirmed by our data (Table 3 and Figure 4). In olive and laurel, a correlation between HSC and refilling was apparent, and no diurnal change of stem starch content was observed when xylem repair was absent (i.e., in May and October for olive and in January for laurel). However, in carob, the starch content of HSC was always low except in May. Moreover, in this species HSC did not change diurnally, despite the occurrence of recovery of embolized conduits. There are four possible explanations for these findings: (i) starch is not involved in the process of refilling, at least in some species; (ii) polysaccharides other than starch are involved in refilling in carob; (iii) starch deposits are much bigger than the amount of sugars actually required to generate the osmotic pressures sufficient to refill embolized conduits; or (iv) the method used to estimate the changes of starch content is only semi-quantitative and does not permit exact quantification of starch amount, so that it cannot be excluded that small changes of starch content occurred also in carob wood parenchyma cells.
Secchi et al. (2011) have reported that upon embolism formation, the metabolic pathways for the transcription of monosaccharide are down-regulated, while those for disaccharides and starch are up-regulated. Nevertheless, the metabolic coordination between carbon supply, growth and storage in trees is still largely unknown, and carbon supply (via photosynthesis) and overall demand (including probably the refilling process) are often not synchronized (Sala et al. 2012).
Based on our present results, we can suggest that the presence of starch in xylem parenchyma cells does not necessarily imply an ability to repair embolized conduits. However, starch seems to be a pre requisite for refilling, at least in some species like O. europaea and L. nobilis.
Functional links between ionic effects and embolism repair
The mechanisms of embolism repair and ion-mediated compensation of Kstem loss were apparently activated by the three species on different temporal scales to optimize the water transport from soil to sites of photosynthesis even under moderate drought stress conditions. In accordance, LSCstem values, as measured in planta with the EFM, remained always constant throughout the study period, despite substantial accumulation of embolism during some months (Figure 6). In fact, despite (i) the rainfall in Messina during the summer of 2012 being higher than the mean recorded from 1971 to 2000 (120.6 mm versus 58.3 mm; source: Italian Air Force Meteorological Service) and, as a consequence, (ii) the lowest ψPD values being higher than −0.1 MPa in all three species (Table 1), the dynamic water stress developing during the daytime was sufficient to significantly impair xylem function (Figure 4). However, despite standard values of PLC >60–70% being recorded in some months in our species, the actual PLC estimated by perfusing solutions mimicking native [K+] (i.e., loss of Kstem in planta) was never higher than ∼50%.
According to data reported in the literature, the ψL at the turgor loss point between May and September is about −2.1 MPa in C. siliqua, −3.1 MPa in O. europaea and −2.6 MPa in L. nobilis (Lo Gullo and Salleo 1988, Correia et al. 2001). In C. siliqua, midday ψL was constantly higher than −1.7 MPa even in the driest period, and no reduction of gas exchange was observed (Table 2). In olive, the most negative values of ψL (about −2.9 MPa) were recorded in July. Such ψL values are common for olive even under moderate water stress (e.g., Guerfel et al. 2009, Quero et al. 2011). Nevertheless, also in this species, the turgor loss point was never reached, and gL was reduced by only ∼30%. Moreover, in laurel the minimum ψL measured was about −1.87 MPa, (i.e., still above zero turgor) and gas exchange rates decreased by ∼30%. In other words, data reported in the present study suggest that PLC values of ∼50–60%, although suggesting substantial xylem dysfunction, were still compatible with maintenance of adequate leaf water supply and relatively high gas exchange rates.
Previous studies have suggested ionic effects and embolism repair as useful mechanisms to face drought and salt stress and to optimize the delivery and use of resources such as light and nutrients (Trifilò et al. 2008, 2011, 2013, Nardini et al. 2010). However, the present study is the first in which the regulation of xylem water transport is shown to depend on a strongly coordinated integration of both mechanisms on daily and seasonal time scales. This reinforces the idea that an efficient transport system must be able to modulate water transport as a function of micro-environmental changes (Nardini et al. 2012, Zwieniecki and Secchi 2012). This physiological trait could probably play an important role in shaping vegetation composition, distribution and plant survival in response to extreme climatic events. According to our current understanding, global climate changes are the cause of widespread forest decline (e.g., Allen et al. 2010, McDowell 2011, Choat et al. 2012). The impact of extreme droughts on vegetation is predicted to be higher in fragile biomes such as Mediterranean-type ecosystems (Martínez-Vilalta et al. 2002, Matusik et al. 2013). In turn, a differential impact of severe drought events on vegetation could be expected on local scales as a function of the species-specific ability to regulate Kstem in response to the risk of hydraulic failure. Understanding different species' ability in xylem transport modulation could be useful in predicting the impact of drought events on vegetation. However, more research is needed to clarify the occurrence of both refilling and ionic effect mechanisms in larger plant species data sets, as well as the eventual correlations between vulnerability to cavitation, xylem transport regulation and safety margins and, last but not least, the possible variability in the ability to regulate water transport at different drought stress levels.
Conflict of interest
None declared.
Funding
This study was financed by University of Messina (Atheneum Research Project).


![Mean values ± SD (n = 8) of xylem sap [K+] as measured in morning (black columns) and midday (white columns) branches of (a) C. siliqua, (b) O. europaea and (c) L. nobilis in 2012. Different letters indicate significant differences for Tukey's pairwise comparisons (P < 0.001).](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/treephys/34/2/10.1093/treephys/tpt119/2/m_tpt11901.jpeg?Expires=1716567478&Signature=gu2i4CEEO5T-F-s0EknUSXnDbuXeRriz2EC-6VkgjXET6nvwZSV86ZvBl7dO4aLycveC57ARu0ji~dQMaTlyqteySG4Y1p8Emzx9Qghik5SqfJ~x-o~H1oKeO87sicqraZ7T4BJzKd3G-v4qAktyASMI3J-vxluaeDG0FVkcsdN4X14G7nycuDjccrF2MVUS3t4GLxjBxtOnI7eEjVTaVqNg5KkEXzrSYyghYyVUq4xVj~VBPXV8opXpOB~05x7~Geal-FirKaLmVYHyrzAtwGUx4EYpRdptqbCccEgtD7gIYJtbmA6HXr4sxxpgbB4TvYJdJinnjlrfwZrc~OP5cQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Means ± SD (n = 8) of the percentage ΔKstem of embolized samples as induced by a reference solution enriched with [K+] similar to the concentration recorded in planta compared with the reference solution. Different letters indicate significant differences for Tukey's pairwise comparisons (P < 0.001). Grey and dashed grey columns refer to midday and morning samples, respectively.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/treephys/34/2/10.1093/treephys/tpt119/2/m_tpt11903.jpeg?Expires=1716567479&Signature=u65UbJOzU~NP2oss3WXJf0YJdefeCe0Vo2GURE9alMIUQUR7GidsfB2g98EagM6C-3oltIu-J168tnz8ed971MIWcWks6gVhvGHFFHCqFYxQ5UsN48-NtouVUAPIdkR8Q5SlpnNvLY78Yx7Btzqg~UBDiBQ5SFzBSfslny-usL8kU8aC3huOrdFi5NdERwIhz9PdB9uUkK~wkgNU5Ym4fhBCca6b2OFmHlWB3mJWlCFJ6ekvIVgov6c1jWmHfF3rZSvx9xSA203nyRp0885VuWemc5pf-RAJTC1El-88YMtaFF2D2qolkggb0Nd1aN4ovSlYimogiyTpAL~usJKo8g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Means ± SD (n = 8) of the PLC as measured in (a) C. siliqua, (b) O. europaea and (c) L. nobilis during 2012. Percentage loss of stem hydraulic conductance was obtained after perfusion with a solution containing 3 mM [K+] or after perfusion with a solution containing potassium concentrations corresponding to [K+] values as measured in planta (i.e., native [K+]). White and dashed white columns refer to midday samples, grey and dashed grey columns to morning samples. Different letters indicate significant differences for Tukey's pairwise comparisons (P < 0.001).](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/treephys/34/2/10.1093/treephys/tpt119/2/m_tpt11904.jpeg?Expires=1716567479&Signature=seYnOWLqM5tSqL2to7vvMHU1ywbSsIB96pDSn28kqEUHCtcl51BoSbvu1alsOM3XMZkkF4OkwHwMkAgsKJWjagdkbJ6HDqalgcTyLQxIJ5Zz3RE3BSvsCpprfW-m7CkXZT3mwtxn778qjY7xuV7bc~CLvLaJG6Et1jGlA8738Ep1eF1D3eTv~SbcChGj8ri-dVAbID7C-1MxSaH5EVS~EpFjETzmPvdTszoY-2QPp0C0Qwfb01qHKjY-XLSnqQ632Tcpu6x1qsOrPopHjDlzrRGM~EwQd5Sly5YYSgqp~V3bycdNZybhmv1IaZctHZZZTTJLnT6gabA9Pm3XOeabzA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![(a) Means ± SD of ΔKstem (%) recorded in fully hydrated samples as a function of means ± SD PLC at 3 mM [K+] for the three species; (b) means ± SD of ΔKstem (%) recorded in fully hydrated samples as a function of means ± SD of FVM for the three species. The regression line, R2 and P values are reported.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/treephys/34/2/10.1093/treephys/tpt119/2/m_tpt11905.jpeg?Expires=1716567479&Signature=c3KbtFW9T6jH1~RR977qtABKjjMf5BnB1qJvHHG8T0emj-U0XLBbBSBVWS40gKPRd0WyHWiSLGFDzeY4fIbiFxiD~fv8y5jXikwMiqEEgEgWG4sFyfUiwJh4~5LO28pEkVH3avCjMxunkI3ilNR-vmy3~60j4xmwWthoPoP3PVDF1DQwDEPXHRd3Dd61Mf4Z4Nk4C1cWFLKyWE30CqFmtf30zWtSLfO~J8YlRB-5igqV-EWirWLcLde6sQsbWhKgtkTNEZB3WqxLe2yd0y-d2acOvHoRUYqLh6azns2aDB3O4GHJf2W8VKxbYrhJs7IdPZ58fVlcOl5AT1HMw-ypKg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)