-
PDF
- Split View
-
Views
-
Cite
Cite
S. Rocha, M. Branco, L. Vilas Boas, M.H. Almeida, A. Protasov, Z. Mendel, Gall induction may benefit host plant: a case of a gall wasp and eucalyptus tree, Tree Physiology, Volume 33, Issue 4, April 2013, Pages 388–397, https://doi.org/10.1093/treephys/tpt009
Close - Share Icon Share
Abstract
Gall-inducing insects display intimate interactions with their host plants, usually described as parasitic relationships; the galls seem to favor the galler alone. We report on a case in which the presence of the galls induced by Leptocybe invasa Fisher & LaSalle (Hymenoptera; Eulophidae) benefit its host plant, the river red gum Eucalyptus camaldulensis Dehnh. Field observations showed that E. camaldulensis plants infected by this gall wasp were less susceptible to cold injury than neighboring conspecific plants without galls. In the laboratory, frost resistance was compared between galled and non-galled plants which were both divided into two subgroups: cold-acclimated plants and plants that were non-acclimated. Galled plants displayed higher frost resistance than the non-galled ones, and the differences were higher in non-acclimated plants compared with acclimated ones. Physiological changes in host plant were determined by chemical analyses of chlorophylls, proteins, soluble sugars and anthocyanin contents. The results showed higher values of all physiological parameters in the galled plants, supporting the hypothesis that the presence of the gall wasp induces physiological changes on the plant foliage, which may in turn increase plant defense mechanisms against cold. Therefore, the toll of galling by the herbivore may pay off by the host plant acquiring increased frost resistance. This work provides evidence for physiological changes induced by a herbivore which might have a positive indirect effect on the host plant, promoting frost resistance such as cold acclimation.
Introduction
Organisms interact not only directly but also indirectly with other organisms in nature. While direct interactions among organisms have been comprehensively studied, indirect interactions have only recently received much attention and are now being considered important forces in forming ecological communities (Strauss 1991, Lewinsohn and Prado 2006, Ohgushi et al. 2007, Ohgushi 2008), and interfering in trophic interactions such as in ant–plant (Guimarães et al. 2006), parasite–host (Lafferty et al. 2006) and plant–herbivore (Ohgushi 2005, 2008, Ohgushi et al. 2007) systems.
In many host–parasite interactions, the effects of parasitism may extend beyond the direct negative effect of the parasites on host growth, reproduction and survival (Puustinen and Mutikainen 2001). Parasites can also change host behavior (Maitland 1994), indirectly affect host vulnerability to other consumers (Price et al. 1986, Ramsell and Paul 1990, Linhart et al. 1994) and shape the structure and dynamics of host communities by changing the competitive interactions among host species (Price et al. 1986, Bonsall and Hassell 1999, Cameron et al. 2009). Hosts can have equally complex direct and indirect effects on their parasites (Adler 2003). While the long-term positive effect of defoliation is well documented (e.g., Bryant et al. 1983, Faeth 1986, Feeley and Terborgh 2005), especially with regard to nutrient cycling, there is no evidence in the literature that a plant–insect interaction could affect the host plant both in a direct negative and an indirect positive way. In this work we aim to demonstrate an indirect positive effect of a gall-inducing insect on its host plant. Insect herbivores specialize in many forms, of which gall inducers and their host plants display the most intimate links (Price et al. 1987, Cook et al. 2002, Stone et al. 2002). A gall is a structure composed of plant tissue within which the galler feeds and is distinguished from other insect-generated shelters by the fact that it involves active differentiation and growth of plant tissues (Price et al. 1987, Abrahamson and Weis 1997, Stone and Cook 1998, Cook et al. 2002, Stone and Schonrögge 2003). Gall inducers are found in a wide range of insect families (Cook et al. 2002) and display great specificity with regard to both host-plant species and the galled organ (Mani 1964, Fernandes et al. 1997, Abrahamson et al. 1998, 2003, Nyman et al. 2000, Ronquist and Liljeblad 2001, Cook et al. 2002, Raman et al. 2008). The genus Eucalyptus in its native range sustains a rich fauna of gall-inducing insects (Blanche and Westoby 1995, Blanche 2000). Eucalyptus is also a unique genus in hosting several Eulophidae wasps (Hymenoptera: Chalcidoidea) as gall inducers (Mendel et al. 2004, Kim et al. 2008, Protasov et al. 2008). Several eulophid gall wasps from eucalyptus found their way into other regions outside Australia, and have become invasive species; among them is Leptocybe invasa Fisher & LaSalle (Mendel et al. 2004). In the new habitats, the massive presence of galls induced by L. invasa has drawn attention to the severe damage and consequent economic impact in nurseries and eucalyptus plantations with the river red gum (RRG) Eucalyptus camaldulensis Dehnh. being one of the most susceptible species to this gall wasp (Mendel et al. 2004, Branco et al. 2008, Thua et al. 2009, Jhala et al. 2010).
The adult of L. invasa is very small (1.0–1.4 mm long), and it lays the eggs in the cortex of shoots or the midribs of leaves and may produce two or three generations annually by parthenogenesis (Mendel et al. 2004). The developing larvae form typical galls in the form of distinct swellings on the leaf midribs, petioles and stems on new foliage on trees of all ages (Kim et al. 2008). Heavy galling causes the leaves to warp and in extreme cases it may stunt the growth of the tree (Mendel et al. 2004).
Field observations in Israel on RRG in 2008 showed that the presence of L. invasa could make the plant acquire resistance to cold. If true, this would be a particular case in which the presence of the gall inducer could have a positive indirect effect on the host plant. To our knowledge a positive indirect effect of the herbivore on the host plant has never been reported before.
Gall-inducing insects seem to control gall formation, leading to accumulation of high concentration of nutrients and metabolites in gall tissues. This is achieved by intensifying photosynthetic rates in the galling plant parts (Fay et al. 1993, Stone and Schonrögge 2003) and by acting as sink. Thus, an increase of sugars and amino acids was observed in the gall tissue (Hartley 1998). Our observations of frost resistance on RRG seems to indicate that physiological changes may also occur on the healthy tissues of the galled plant, which may, ultimately, have a secondary positive role in plant survival by reducing the effect of negative factors such as frost.
Based on the L. invasa–RRG system, the present study aims at testing the hypothesis of a positive indirect interaction of the gall inducer on the host plant, expressed in the form of frost resistance. We further analyze the physiological changes induced by the galler on the host-plant healthy foliage as a possible explanation for the acquired frost resistance.
Materials and methods
Field observations
Field observations were conducted during a frost period, in January 2008, in Israel (Bet Dagan). The monthly mean temperature was 5.0 ± 0.9 °C, ranging from −1 to 10.6 °C. Three groups of E. camaldulensis trees, of 7-year-old (n = 7), 3-year-old (n = 3) and 6-month-old seedlings (n = 50), were inspected for the presence of galls and for cold damage. From each tree, 10 branches of each type, with and without galls, were sampled; for seedlings 25 galled and 25 non-galled plants were sampled. The total number of shoots, and the number of shoots affected by frost damage (recognized by leaf necrosis), was recorded for each tree branch and seedling.
Experimental trials
Plant material
Seeds of E. camaldulensis (Lake Albacutya provenance) were collected in Hula Valley, Israel, and sown in a nursery in controlled conditions (20–22 °C and a photoperiod of 14/10 h) at ISA, Lisbon, Portugal. Germination started in February 2011. Six-week-old seedlings were transferred to 120 cm3 containers with a substrate consisting of peat and vermiculite with a ratio of 2 : 1 and fertilized weekly for 9 weeks with a nutrient solution composed of 2 mM Ca(NO3)2.4H2O, 1.6 mM Mg(NO3)2.6H2O and 0.2% commercial fertilizer (Complesal, Bayer Crop Science, Lisbon, Portugal) (v/v). Plants were kept in a greenhouse, and after 5 months of growth they were transferred to 4 l containers filled with the same substrate and watered daily.
At the end of July 2011, the plants were divided into two groups: (i) 53 plants (which will be exposed later to L. invasa) and (ii) 51 plants (which will be serving as the control). Each group was placed separately inside a field cage insectarium (2.42 m wide × 2.45 m deep × 3.70 m high) separated from the exterior by a net with a mesh of 1 × 1 mm to prevent penetration by insects. The plants were watered on average 4 times a week until field capacity. In both cages plants had similar temperature and humidity. The temperature inside the cages ranged from 24.3 to 29.6 °C and the relative humidity from 40 to 70% during the experimental period.
Leptocybe invasa inoculation
In August, branches of Eucalyptus tereticornis Sm. and Eucalyptus saligna Sm. × Eucalyptus rudis Endl. heavily infested with L. invasa were collected from a young stand (Herdade de Espirra, Pegões, Portugal). All the branches brought from the field were carefully examined and washed to avoid the presence of other insects. Branches with mature galls of L. invasa were then selected and placed inside the test cage of group A, to ascertain the exposure of the plants to the wasp attack. Branches were put in a pot filled with water to prevent dehydration and substituted by new ones twice weekly. Gall formation was observed and their number per plant and per leaf (midrib vs. petiole) was recorded ∼7 weeks after wasps started to emerge.
Acclimation
When galls were ∼90 days old, corresponding to the stage of mature larvae and pre-pupae (Protasov et al. 2008), 12 plants from the test group (galled plants) and 12 from the control group (non-galled plants) were selected for the frost resistance measurement. At this time plants were ∼9 months old. The selected plants displayed approximately the same size, ca. 1 m height, and a similar number of branches and branch development. The 12 plants from each group were randomly divided into two sub-groups: six for cold acclimation and the other six were kept at ambient temperature. Therefore, 12 plants (6 inoculated with L. invasa and the other 6 from control ones) were placed in a chamber for artificial acclimation (Fitoterm 3600, Aralab, Oeiras, Portugal). The acclimation consisted of a period of 1 week with a gradual temperature decrease (2 °C per day) from 17/5 °C to 10/ − 2 °C (day/night) and a photoperiod of 11/13 h (day/night). The acclimation period and selected temperatures were based on previous works (Costa e Silva et al. 2007; Shvaleva et al. 2008) and preliminary experiments. All containers were protected at the top with plastics and styrofoam, and soil temperature was measured every day to make sure that it was >9 °C, to prevent any damage to the roots due to low temperatures. Twelve other plants (six inoculated with L. invasa and six non-inoculated) were placed outside (non-acclimated) during the same week (mean temperatures 22/15 °C day/night). During that period, all plants (acclimated and non-acclimated) were watered as needed.
Frost resistance
Frost resistance was assessed by submitting totally expanded leaves to different freezing temperatures ranging from −3 to −8 °C, according to Almeida et al. (1994). This range of temperature is in agreement with the lowest temperatures (−8 °C) on record near Lake Albacutya, Victoria (Eldridge et al. 1994). From each plant group (non-galled acclimated, galled acclimated, non-galled non-acclimated and galled non-acclimated) and for each tested temperature 18 leaves were collected (6 plants × 3 leaves per each plant). Leaves were then wrapped in aluminum foil (to prevent water loss and temperature fluctuations) and subjected to a specific temperature in a controlled temperature chamber (Fitoclima 750E, Aralab). In the case of galled plants, only leaves without galls were used for both frost treatment and chemical analysis, to ensure that we were analyzing an indirect effect. Five target temperatures were tested: −3, −4, −5, −6, −7 and −8 °C, based on preliminary freezing tests. For each temperature, 18 replicates were tested per treatment and per type. A controlled freezing program followed a constant cooling and thawing rate of 4 °C/h and a 2 h exposure to different target freezing temperatures. The temperature inside the chamber was recorded with a sensor to verify any deviations from the programmed temperatures. The degree of damage sustained by the leaf tissue results from membrane injury and was evaluated by the extent of leaching of cell electrolytes from discs (Almeida et al. 1994, Eldridge et al. 1994). After the cold treatment, three discs (Ø 1 cm) from each leaf were carefully cut and placed in a Pyrex vial containing 15 ml of deionized water. The vials were capped and placed in a 25 °C water bath during 24 h; after this period the electrolyte conductivity (C1) was measured with a conductivimeter (K220 Consort, Turnhout, Belgium) to the nearest μS cm−1. The samples were then boiled in an autoclave at 120 °C for 10 min before being returned to the water bath at 25 °C. After 12 h, the electrolyte conductivity of the dead tissue (C2) was measured. The relative conductivity at each target freezing temperature (Rt) was obtained as Rt = (C1/C2) × 100. The Rt data were used to define the lethal temperature because Rt values for each seedling were directly correlated (r2 = 0.98) with the degree of leaf damage (Hallam and Tibbits 1988, Almeida et al. 1994). The lethal temperature was defined as the temperature resulting in 50% loss of cellular electrolytes (T50). The temperature at which there is 50% plant mortality (LT50) was estimated from linear interpolation as described by Hallam and Tibbits (1988) and Tibbits and Reid (1987), allowing us to estimate the frost resistance of both treatments (acclimated and non-acclimated) and both types (galled and non-galled plants).
Chemical analyses
For the four groups of plants (acclimated and non-acclimated, galled and non-galled), we measured the following chemical parameters for healthy non-galled leaves: chlorophylls, soluble sugars, soluble protein content and leaf pigments with red light absorbance. Chemical analyses were expressed on the leaf area basis rather than fresh mass to exclude the possible effect of water content.
Chlorophylls
Chlorophylls (total, a and b) were measured by the Arnon (1949) method (modified) using fresh leaf tissue. Two discs (Ø 1 cm) were cut from each of three fully expanded leaves per plant (for the four groups). These were placed in test tubes with 1 ml of ethanol (80%) and centrifuged for 5 min at 13,000 g under low light conditions. The absorbance was measured at 663 nm (chlorophyll a) and 645 nm (chlorophyll b) with a spectrophotometer (U 2001, Hitachi, Tokyo, Japan).
Soluble sugars
Soluble sugars were extracted from leaf discs (3.14 cm2) with 80% (v/v) ethanol at 80 °C for 20 min. The quantifications of glucose, fructose (hexoses) and sucrose were done by adding 20 μ l of ethanol extract in 1 ml of reaction mixture containing: 0.1 M imidazole (pH 6.9), 1.5 mM MgCl2, 1 mM ATP, 0.5 mM NADP+, 0.5 mM hexokinase (EC2.7.1.1). After a 5-min incubation, the following enzymes were added: 0.1 U glucose-6-phosphate dehydrogenase (EC1.1.1.49) for glucose quantification, glucose-6-phosphate isomerase 0.1 U (EC 5.3.1.9) for fructose quantification and finally invertase (EC 3.2.1.26) for sucrose quantification. Hexoses and sucrose were measured in the ethanol extract spectrophotometrically by following NADPH oxidation at 340 nm. Soluble sugars in leaves were assayed by an enzymatic method based on the methodology described by Stitt et al. (1978).
Soluble protein content
For total soluble protein, the samples, leaf discs (3.14 cm2), were ground to a fine powder in a mortar, homogenized in 1 ml of extraction buffer containing 50 mm Tris–HCl pH 8, 20 mM MgCl2, 50 mM β-mercaptoethanol, 2 mM benzamidine, 2 mM phenylmethylsulfonyl fluoride and 2.5% Tween 20. Extracts were clarified by centrifugation (13,000 r.p.m. for 10 min) and the supernatant was immediately assayed for protein quantification according to the method of Bradford (1976), using bovine serum albumin as the standard.
Leaf pigments at red light absorbance
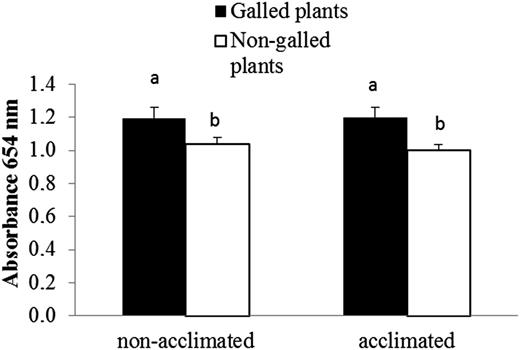
For leaf anthocyanin quantification we used acid dimethylformamide (DMF) (0.1 N HCl solution with DMF) and the samples were allowed to extract for 24–36 h. The extraction is verified when the leaf disk looks basically white (i.e., no color left), and the solution is red. The extract was read in a spectrophotometer (U 2001, Hitachi, Tokyo, Japan) at 654 nm. The absorbance at 654 nm (red light) was considered as an indicator of the plants’ anthocyanin contents. Nevertheless, other compounds may absorb at 654 nm which would be included in this analysis.
Statistical analysis
A General Linear Model procedure, using a Poisson distribution, was used to model the number of twigs affected by cold on galled and non-galled eucalyptus trees/branches observed in the field (Israel). The variable age was considered as a covariate. The values are presented as means ± SE. The intensity of cell damage was analyzed by two-way analysis of variance (ANOVA), considering the factors plant type (galled and non-galled plants) and treatment group (acclimated and non-acclimated plants), with temperature as a covariate.
Two-way ANOVA was used to test the effects and interactions between treatments (acclimated and non-acclimated) and types (galled and non-galled) for chlorophyll, protein and absorbance at 654 nm. Total sugar content was tested for non-acclimated plants with one-way ANOVA. Tests of normality and homogeneity of variances were performed by the Kolmogorov–Smirnov and Levene statistics, respectively. Differences were considered as statistically significant at P ≤ 0.05. Data are presented as means ± SE. All analyses were performed using the SPSS statistical package 20.0 (SPSS 2011).
Results
Field observations
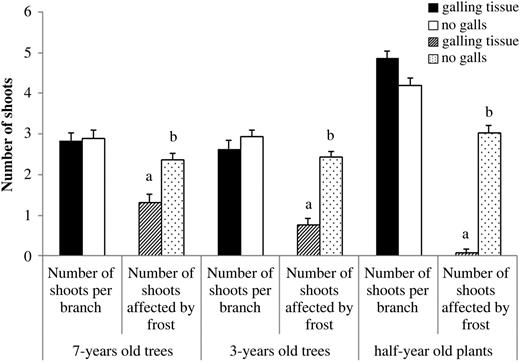
Observations of 7-year-old, 3-year-old and 6-month-old E. camaldulensis trees revealed significantly less damage by frost on galled branches/plants in comparison with non-galled ones; Wald χ2 is 310.12, d.f. = 1, P < 0.001 (Figure 1). The number of shoots affected by cold damage also decreased significantly with the covariate age; Wald χ2 is 69.52, d.f. = 1, P < 0.001. This demonstrates more cold damage on the (younger) 6-month-old trees (Figure 1).
The mean (±SE) number of shoots observed per branch/plant and the mean number of shoots affected by frost in galled and non-galled branches of 7-year-old, 3-year-old trees and 6-month-old plants of E. camaldulensis growing in the coastal plain of Israel.
Experimental trial
Gall inoculation
As planned, the plants from the control group were completely free of any type of galls. Plants exposed to L. invasa had a mean number of galls per plant of 101 ± 10.0 (n = 51). Plants randomly assigned to acclimation treatment had a mean of 120 ± 13.9 (n = 12) galls per plant and non-acclimated plants had a mean of 82 ± 10.4 (n = 12).
Frost resistance
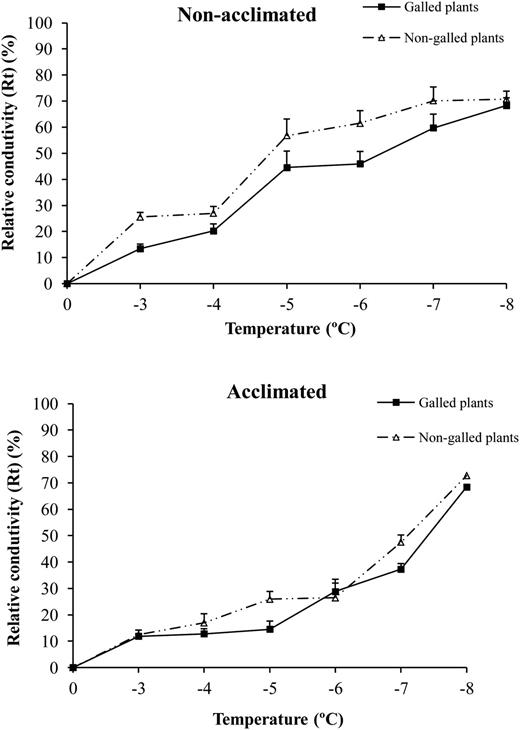
For all plants, damage increased with decreasing temperature, thus representing intensifying cold shock (F1,1 = 434.15; P < 0.001). Overall, damage intensity was significantly lower on galled plants when compared with non-galled plants (F1,1 = 18.07; P < 0.001), thus revealing higher tolerance to frost. This effect was particularly evident on the non-acclimated treatment (Figure 2). As expected the frost resistance increased steeply and was significantly higher on acclimated plants when compared with non-acclimated ones (F1,1 = 74.48; P < 0.001). Interaction term treatment × type of plant was not significant (F1,401 = 1.85; P = 0.175).
Mean (±SE) relative conductivity (Rt) (%) caused by cold treatment in galled and non-galled E. camaldulensis young plants in non-acclimated plants (top) and acclimated plants (bottom) at different cooling temperatures.
The LT50 value for non-acclimated galled plants was −6.5 °C and for non-acclimated non-galled ones it was −4.7 °C, thus a difference of −1.8 °C. The difference was not so high for the acclimated plants though; galled plants still had lower LT50 (−6.9 °C) than non-galled ones (−6.6 °C), with a difference of −0.3 °C.
Chemical analysis
Chlorophylls
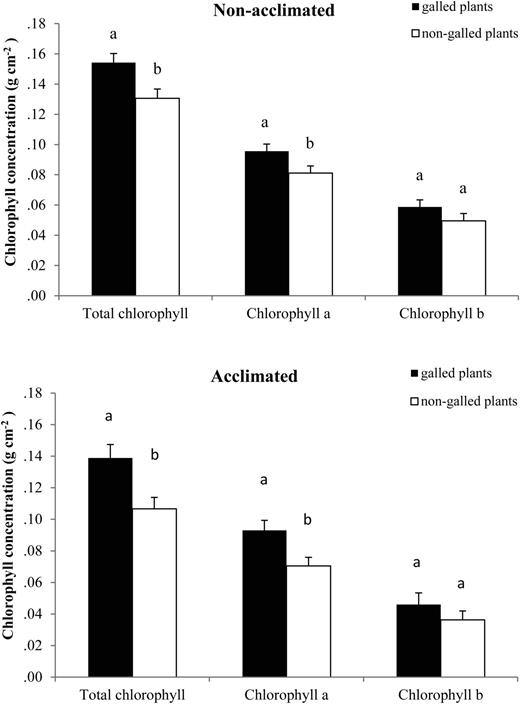
Galled plants showed higher total chlorophyll content than non-galled ones on both acclimated and non-acclimated treatments (Figure 3). Total chlorophyll content differed significantly between the two types of leaves, from plants with galls vs. plants with no galls (F1,52 = 15.57; P < 0.001). No interaction was found between acclimation treatments and types of plants (F1,52 = 0.369; P = 0.546). Chlorophyll a was significantly higher in galled plants (F1,52 = 12.07; P < 0.001), observed in both non-acclimated and acclimated plants (Figure 3). No significant differences were found in chlorophyll b content between galled and non-galled plants in both acclimated and non-acclimated plants (F1,52 = 2.91; P = 0.094).
Chlorophyll content (g cm−2) of healthy leaves of E. camaldulensis from galled and non-galled plants in non-acclimated (top) and cold-acclimated plants (bottom) (data are means ± SE).
Soluble sugars
The sugar content of galled plants in non-acclimated plants was 0.055 ± 0.02 μ mol cm−2 and that for non-galled plants was 0.049 ± 0.01 μ mol cm−2. No significant differences were found between the two (F1,34 = 0.104; P = 0.749). Values of acclimated plants were not possible to determinate since a conservation problem of the vegetable material occurred making it unviable for sugar content analyses.
Soluble protein content
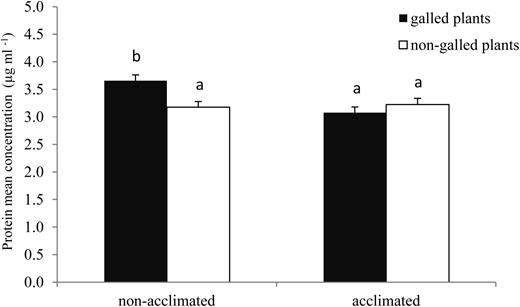
For total protein contents, the interaction between acclimation treatment and types of plants was significant (F1,64 = 8.54; P = 0.005). The mean estimates and their 95% confidence intervals, for each factor × level, revealed no significant differences between groups, except for the galled non-acclimated plants, which showed significantly higher protein content than the three other groups (Figure 4).
Total protein content (μg ml−1) of healthy leaves of galled and non-galled E. camaldulensis plants, from cold non-acclimated and acclimated plants (data are means ± SE).
Leaf pigments at red light absorbance
Absorbance at 654 nm was significantly higher on galled plants when compared with non-galled ones (F1,57 = 8.934; P = 0.004). The same pattern was observed for both acclimated and non-acclimated treated and non-acclimated treatments (Figure 5). However, significant differences were not found between cold-acclimatments (F1,57 = 0.084; P = 0.773).
Absorbance (654 nm) of healthy leaves of galled and non-galled E. camaldulensis in cold non-acclimated and acclimated plants (data are means ± SE).
Discussion
We provide here evidence of the indirect positive effect of a gall inducer on the host tree tolerance to cold. Field observations on E. camaldulensis of different ages showed that plants/branches with L. invasa galls were less injured by frost than non-galled ones. As expected, cold susceptibility was higher in young plants, and thus, the positive effect of the gall former in inducing plant frost resistance was particularly evident on 6-month-old young seedlings. Frost resistance laboratory assays also showed that the presence of the galls decreased the damage caused by cold in a range of temperatures that varied from 0 to −8 °C. This result was observed for both acclimated and non-acclimated plants, when comparing galled and non-galled plants. The LT50 difference between galled and non-galled was −1.8 °C in the non-acclimated plants and – 0.3 °C in the acclimated plants. As expected, cold-acclimated plants also showed less freezing damage (lower LT50) than non-acclimated ones as observed in Eucalyptus globulus Labill. (Almeida 1993, Almeida et al. 1994). Although the observed LT50 differences seem to be relatively small, they might represent significant effects on plant fitness as found in other studies with different eucalyptus species. According to Almeida (1993), in a study with different E. globulus genotypes, 0.5 °C difference was enough to kill all the plants or leave them unharmed. Kirkpatrick (1975) in a study of cold resistance in E. globulus found that differences of 1 °C caused a duplication of damage. Also, Tibbits and Reid (1987) found that a decrease of 1.2 °C caused a variation from 0 to 90% of damage in leaves of Eucalyptus nitens (Deane & Maiden) Maiden in non-acclimated plants. With these results, we may infer that the activity of the gall inducer indirectly led to a positive and similar effect to cold acclimation, even though this effect was slightly superior. Similarly to the effect of cold acclimation (Almeida 1993, Almeida et al. 1994), a positive effect on the galled plants is expected owing to a decrease in the lower temperature threshold causing leaf damage, thereby increasing the length of the growing period and enabling higher plant productivity.
In the present experiments, the presence of the galls not only increased frost resistance, but also induced significant changes in the physiology of the plant foliage: proteins, light absorbance and chlorophyll measures differed significantly between galled plants and non-galled ones. Conspicuous changes in host plant physiological processes caused by insect galls have been observed in other studies (Hartley 1998, Fernandes et al. 2010). As in our case (and others, e.g. Mani 1964) physiological changes were observed both locally, and spread systemically to nearby growth. Some studies demonstrate that gall induction by insects reduces photosynthetic rates in plants (Larson 1998, Yang et al. 2003, Florentine et al. 2005, Albert et al. 2011). However, other studies (Fay and Hartnett 1991, Fay et al. 1993) showed increased leaf photosynthesis in galled plants compared with non-galled ones. Plants can compensate for the adverse effects of herbivores through compensatory photosynthesis, defined as an increase in photosynthetic rates on damaged plants relative to undamaged ones (Nowak and Caldwell 1984). Thus, compensatory photosynthesis could explain the higher chlorophyll and leaf pigments contents observed in this work on galled plants rather than on non-galled ones.
In our study, galled plants showed higher total protein content compared with non-galled ones; thus a systemic effect is inferred. Other studies have found increased protein content in galled plants, but especially in the nutritive gall tissues on which the insect feeds (Paclt and Hassler 1967, Schönrogge et al. 2000, Allison and Schultz 2005). In a study by El-Akkad (2004) with Populus nigra L. and the gall-forming aphid Pemphigous populi Courchet, small differences in the protein content between non-galled and galled leaves were observed. However, El-Akkad (2004) noticed the presence of specific proteins in the galled leaves which were absent in the non-galled leaves. Also, Schrongge et al. (2000) showed that cynipid gall formation might involve the expression of specific proteins in galled tissues. Plants usually respond to stress situations, like drought, cold, and herbivory, increasing their protein contents (Levitt 1980, Thomashow 1998, Wang et al. 2003, Uemura et al. 2005, Beck et al. 2007), and specifically low-molecular-weight nitrogenous compounds such as proline (El-Akkad 2004, Beck et al. 2007, Ruelland et al. 2009). It would be valuable for our study to perform the determination of such specific proteins which might have ensured such a protective effect on the affected plants.
Absorbance of red light (654 nm) was higher on galled plants. Our results are in agreement with previous studies that reported physiological alterations due to gall-forming insects. Foliar anthocyanin has been implicated in eucalyptus frost resistance by Costa e Silva et al. (2007) and plant resistance to herbivory (Coley and Kursar 1996, Close and Beadle 2003). Also, Stone et al. (2001) reported a correlation between insect herbivory and foliar anthocyanin level in E. saligna and Eucalyptus paniculata Sm., which increased in insect-affected foliage during winter.
No differences in sugar content were found between plants galled by L. invasa and non-galled ones. These results may be the outcome of low numbers of replicates and the cumulative errors involved in the determination of these compounds. An increase in sugar content in galled plants seems to be beneficial to the insect once it represents a food source to the feeding larva (Hartley 1998, Yang et al. 2003). Furthermore, it is generally accepted that increased concentrations of compatible solutes result in a decrease in the extent of freeze-induced osmotic dehydration from cells (Levitt 1980), which agrees with the results of numerous studies involving frost resistance (Levitt 1980, Almeida et al. 1994, Leborgne et al. 1995, Uemura et al. 2005). Some studies showed similar responses of plants subjected to freezing temperatures and water deficits, suggesting that frost resistance and drought resistance mechanisms share the same pathways (Sung et al. 2003, Atkin et al. 2005, Costa e Silva et al. 2007). Among members of the genus Eucalyptus, E. camaldulensis is considered the most widely distributed species in its native area, ranging from north to south-western Australia (Eldridge et al. 1994). According to the present study, the association with the gall-former might improve the survival of the host plant in extreme temperature regions. Thus this insect–plant interaction may permit a wider distribution of the species in climatic areas with more severe winter temperatures or with unusual cold episodes in the subtropical regions in Queensland where L. invasa was found (Z. Mendel, M. Branco and J. La Salle, unpublished data). The reproductive activity of the adult wasp overlaps with E. camaldulensis new growth throughout the warm season (Mendel et al. 2004). Thus, when temperature decreases during fall, the galling tissue is completely developed and gall formers have emerged. Therefore, despite the injury caused by L. invasa to young trees, the gall wasps, particularly if at low density, as occurs in its native range (Z. Mendel, M. Branco and J. La Salle, unpublished data), may have an overall compensating positive effect by protecting trees during harsh winter periods.
Gall-forming insects are sensitive to small differences in host-plant physiology, chemistry, development and phenology, and as a result, gall inducers may discriminate among closely related species of host plants (Floate et al. 1996, Abrahamson et al. 1998). Leptocybe invasa develops on E. camaldulensis and other members of the subsection such as E. tereticornis (Mendel et al. 2004, Kim et al. 2008, Protasov et al. 2008). In this work we report a situation that might be considered an ambivalent relationship ranging between a parasitic and a mutualistic interaction. Thus, as should be anticipated the interactions between the gall inducer and its host plant display both conflicts and benefits.
Our study demonstrates that the presence of the gall wasp L. invasa in its host plant E. camaldulensis induces a positive indirect effect that increases frost resistance. The relation between the gall-forming insect and its host plant was usually described as parasitic whereas positive effects were only observed in favor of the gall wasp. The biotic stress as reported here has not been shown until now, and so these results are original and new and allow us to infer that this parasitic relation may have overall benefits for the host plant when the biological balance of the galler population is not disturbed. The underlying mechanisms involved in this process have not been clarified in this work and subsequent research should be conducted in order to understand which particular genes or proteins are expressed by the galler. This would require a complete new work with molecular tools.
Conflict of interest
None declared.
Funding
This study was supported by the PTDC/AGR-CFL/111877/2009 project.
Acknowledgments
We are grateful to Ian Dodkins for English editing; to Carla Faria for sowing and nursery care of E. camaldulensis seedlings, to Ana Rodrigues for technical support and to Ana Vale for laboratory assistance.